A new Apple Watch “anxiety vs arrhythmia” classifier matters right now because palpitations are one of the most common, most confusing symptoms in modern medicine, and wearables have turned that confusion into a constant stream of data. If the watch can triage stress-driven episodes from dangerous rhythms, it reshapes care, cost, and trust.
Why Apple Watch Anxiety Vs Arrhythmia Became A High-Stakes Question?
Palpitations sit at an uncomfortable intersection of cardiology and mental health. They are common, scary, and often ambiguous. A racing heart can be a panic episode, too much caffeine, poor sleep, dehydration, a medication side effect, or an arrhythmia that raises stroke risk. In a clinic, sorting that out is already hard. On a wrist, where signals are intermittent and context is imperfect, it becomes harder still.
The growth of smartwatches turned this into a mass-market problem. Millions of people now carry a device that can detect irregular rhythms, capture single-lead ECGs, and nudge them to “pay attention” to their heartbeat. That is progress, but it also changes behavior. People who might have ignored a brief flutter now watch their pulse like a stock chart. Some feel reassured. Others feel trapped in a loop of checking, worrying, and seeking confirmation.
This is where the “anxiety vs arrhythmia” framing becomes more than a catchy headline. It is a proxy for a broader shift in healthcare: moving from diagnosing disease to managing uncertainty at scale. A watch does not need to perfectly diagnose panic disorder or label every arrhythmia subtype. What it must do, if it wants to be clinically useful and psychologically safe, is reduce the cost of being wrong.
There are three forms of “wrong” that matter.
First, a false alarm. A watch flags a dangerous rhythm when the user is actually experiencing a stress response or sensor artifact. That can trigger emergency visits, costly testing cascades, and long-term health anxiety.
Second, false reassurance. A watch downplays an episode that is actually clinically significant. That can delay care, especially for people who trust devices more than their own symptoms.
Third, ambiguous outputs. The watch provides a warning that is technically accurate but practically unclear, forcing the user and clinician to interpret it without enough context. This is the silent driver of workload. Ambiguity creates messages, calls, extra visits, repeat tests, and a lingering sense that the body is unpredictable.
Apple’s position makes this particularly consequential. The Apple Watch has been one of the most influential consumer medical-adjacent devices in history, partly because it worked with regulators early and partly because it built a workflow around user education, onboarding, and boundaries. The next leap is not just “more detection.” It is smarter triage that lowers unnecessary panic while keeping true danger visible.
Key statistics that frame the stakes:
- In the Apple Heart Study, about 0.52% of participants received an irregular pulse notification in a very large cohort, suggesting Apple’s design is tuned to avoid frequent alerts.
- Follow-up analyses of notified participants found many did not have AFib on confirmatory monitoring, but a significant portion had other incidental arrhythmias, which creates a real-world triage challenge.
- Updated U.S. estimates suggest atrial fibrillation affects at least about 10.55 million adults, far higher than older projections, which raises the potential value of screening.
- Anxiety disorders remain among the most common mental health conditions worldwide, and the post-pandemic era has intensified attention on stress, sleep, and autonomic health.
- The FDA’s January 2026 messaging emphasized clearer exemptions for low-risk wellness tools while maintaining scrutiny for disease-claiming features, shaping how “anxiety vs arrhythmia” can be marketed and validated.
How We Got Here: Apple’s Path From AFib Screening To “Guardian” Health Algorithms?
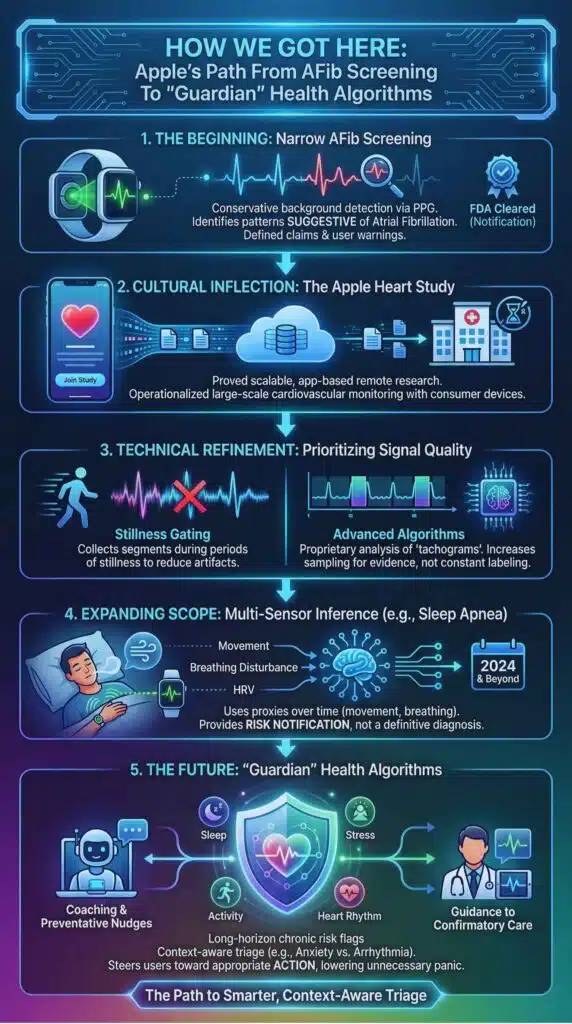
Apple’s heart story did not begin with a promise to diagnose arrhythmias. It began with a narrow claim: identify pulse irregularity patterns suggestive of atrial fibrillation in a background screening mode, under defined conditions, with user-facing warnings about limitations.
That framing mattered. It kept expectations grounded and it gave regulators a concrete target: a software feature analyzing optical pulse data (photoplethysmography, or PPG) to decide whether irregularity might warrant user attention. Apple’s approach also set a template for how consumer health features can scale: build a conservative detector, validate it in large populations, and provide a confirmatory step rather than a definitive diagnosis.
The Apple Heart Study was a cultural inflection point. It used app-based enrollment to reach hundreds of thousands of participants, then connected notifications to a telehealth workflow and confirmatory monitoring. The most underappreciated impact was not the raw accuracy numbers. It was the proof that large-scale, partially remote cardiovascular research could be operationalized with consumer devices. That model now shapes how many digital health trials are designed.
But even early results hinted at the future problem. Irregular rhythm alerts are not the same as clinical diagnoses. Many notified users will not have AFib at the moment a confirmatory patch is worn. Some will have premature atrial contractions or premature ventricular contractions, which can feel dramatic but often require nuanced management rather than emergency response. And many people with palpitations will have normal sinus rhythm, especially when stress or anxiety is driving symptoms. This gap between sensation and diagnosis is exactly what a next-generation classifier must address.
Apple’s own technical descriptions reveal how the company has tried to manage this gap with design choices. The watch does not continuously label every second of pulse data as “normal” or “abnormal.” Instead, it collects segments during periods of stillness and classifies “tachograms” with a proprietary algorithm. If irregularity is suspected, the system increases sampling to gather more evidence, within spacing constraints. That is a subtle but important philosophy: prioritize signal quality and reduce noisy alerts rather than chasing constant detection.
This philosophy also aligns with Apple’s recent expansion into other screening areas that depend on pattern recognition and machine learning. Sleep apnea notifications, for example, use movement and breathing disturbance proxies over time, then provide a risk notification rather than a diagnosis. Whether the next major leap is branded as “Watch X” or simply appears as an algorithm update in a future model line, the direction is consistent: use multi-sensor inference to flag risk, then steer users toward confirmation and appropriate care.
A timeline that shows the arc:
| Year | Milestone | Why It Changed The Wearable Health Narrative |
| 2018 | Regulated, OTC irregular rhythm notifications and ECG positioning | Consumer hardware gained a medical-adjacent lane with defined claims and limits. |
| 2019 | Apple Heart Study results spotlighted large-scale, app-based screening | Proved virtual enrollment and wearable-triggered telehealth workflows could scale. |
| 2020 | Apple published technical details on arrhythmia detection design | Emphasized stillness gating, tachograms, and conservative notification logic. |
| 2024 | Sleep apnea risk notifications gained regulatory traction | Reinforced the “risk notification, not diagnosis” model using machine learning. |
| 2025 | New Watch models leaned harder into “insights” and preventative nudges | The product story shifted toward longer-horizon chronic risk flags and coaching. |
| 2026 | FDA signaled clearer boundaries for wellness vs medical claims | Pushed the industry to choose between faster consumer features and stricter validation. |
This is the context in which “anxiety vs arrhythmia” becomes inevitable. Once a device can detect some clinically meaningful rhythms, it must also answer the user’s real question: “Is this dangerous, or am I just panicking?”
What The New Classifier Likely Changes: From Rhythm Detection To Context-Aware Triage
A watch can be very good at one thing and still fail the user. It can detect AFib with strong performance in the right conditions and still leave people confused when they feel symptomatic but the watch shows “nothing,” or when the watch warns them but confirmation later looks normal. The next algorithmic leap is not just improving AFib detection. It is improving decision-making around uncertain episodes.
An “anxiety vs arrhythmia” classifier, if done responsibly, is less like a diagnosis engine and more like a triage nurse. It attempts to sort episodes into action categories:
- Capture confirmatory ECG now.
- Recheck when still.
- Monitor and log triggers.
- Seek urgent care if red-flag symptoms occur.
- Use a calming protocol and reassess.
The technical route to this outcome is “context fusion.” Rhythm irregularity alone is insufficient. A smartwatch has multiple data streams that can provide context around a heart-rate spike:
- Motion and stillness data can distinguish exercise, restless pacing, and quiet rest.
- Sleep and recovery signals can show whether the user is in a depleted state that can amplify sympathetic activation.
- Trends in heart rate variability can offer a noisy but sometimes useful proxy for autonomic balance.
- Repeated sampling across minutes can show whether a spike is transient and responsive to calming, or persistent and independent of behavior.
The most important point is what the classifier should not do. It should not tell users, “You have anxiety.” That is a clinical diagnosis and a deeply personal label. The safer, more plausible design is to say something like: “This episode appears more consistent with a rapid but regular rhythm and elevated stress response indicators. If symptoms persist or you feel unwell, seek care. Consider an ECG recording if palpitations continue.” That language avoids overclaiming while still offering guidance.
There is also a quality-of-signal issue that matters. Optical sensors are vulnerable to motion artifact. Anxiety episodes often involve fidgeting, pacing, trembling, or altered breathing. In other words, the exact situations where people feel panicked are also the situations where PPG can degrade. A smarter classifier must become better at distinguishing true physiological irregularity from artifact, not just anxiety from arrhythmia.
This is where Apple’s stillness gating strategy becomes strategically important. By design, the watch tries to measure rhythm in conditions where optical signals are more stable. But that also means a panicked person who is moving may not get a clear reading, and may interpret the lack of signal as either reassurance or frustration. The “anxiety vs arrhythmia” experience therefore becomes as much about user interface as it is about machine learning.
A comparative view of what a watch is really trying to separate:
| Episode Type | What The User Feels | What The Watch Often Sees | What A Smarter Triage Experience Would Do |
| Panic or acute stress response | Sudden racing heart, chest tightness, fear | Fast but regular rhythm, motion artifacts, hyperventilation cues | Guide stillness recheck, offer paced breathing, prompt ECG only if palpitations persist |
| AFib-like irregularity | Fluttering, uneven beats, sometimes no symptoms | Irregular pulse segments during stillness | Prompt ECG confirmation and clinical follow-up messaging |
| Benign ectopy (PACs/PVCs) | Skipped beats, thumps, brief runs | May trigger irregular pulse detection but not AFib | Encourage documentation, reduce alarm language, suggest clinician discussion if frequent |
| Supraventricular tachycardia episodes | Sudden fast regular rhythm, dizziness | High rate, regular interval pattern, may be hard on PPG | Encourage ECG capture during symptoms, provide red-flag guidance |
| Sensor artifact or poor contact | Confusing readings, inconsistent pulse | Noisy signal, dropouts | Tell user to adjust fit, recheck, and avoid scary language |
The classifier’s success depends on reducing two downstream harms at once: overt medicalization of stress, and under-recognition of true rhythm risk.
This is why the “anxiety vs arrhythmia” promise is so tempting and so dangerous. If marketing language implies certainty, it invites over-trust. If the experience is too cautious, it fails to reduce anxiety. The winning approach is calibrated humility paired with actionable next steps.
System Ripple Effects: Patients, Clinicians, Regulators, And The Wearables Economy
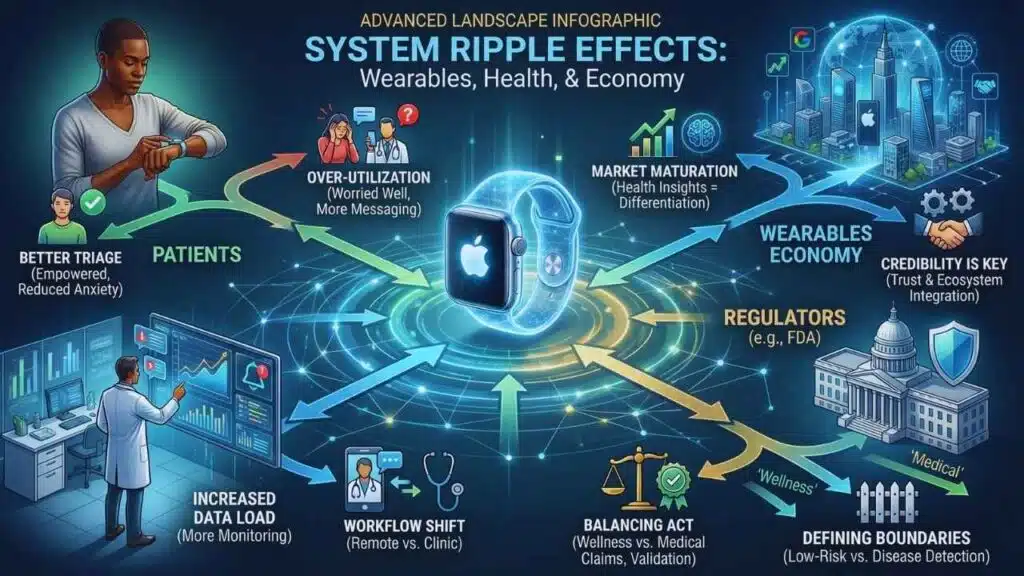
The most obvious beneficiary of better triage is the user. The less obvious beneficiary is the healthcare system.
Healthcare is not overloaded only because of disease. It is overloaded because of uncertainty. Palpitations drive a large volume of emergency visits, primary care consultations, cardiology referrals, and diagnostic testing. A tool that reliably reduces unnecessary visits without hiding true emergencies would be economically meaningful even if it never claims to diagnose anything.
But there is a catch: wearables can also increase utilization. Research in atrial fibrillation populations suggests wearable use can correlate with more symptom monitoring, more anxiety, and more informal healthcare engagement such as messaging clinicians. Even if some of that engagement is appropriate, it represents a workload shift. The device moves part of surveillance from the clinic to the home, but it does not eliminate the clinic’s interpretive role. It can expand it.
That is why “anxiety vs arrhythmia” is really a bet about workflow design. If the watch simply produces more classifications, clinicians get more data and more questions. If the watch produces better triage, clinicians get fewer low-quality contacts and higher-quality confirmatory captures.
The second ripple effect is regulation. The FDA’s January 2026 comments about limiting regulation of low-risk wellness wearables while scrutinizing disease claims create a strategic incentive: companies can ship more features faster if they avoid medical promises. “Anxiety vs arrhythmia” sits right on that boundary. If framed as “stress insights,” it may be treated as wellness. If framed as “we can tell you whether your palpitations are dangerous,” it edges toward clinical decision support and medical device territory.
This matters because the validation burden differs sharply. Medical-adjacent claims require evidence, defined labeling, and ongoing change control. Wellness features can iterate faster, but they risk becoming a confusing gray zone where consumers assume medical-grade accuracy without actually receiving it.
The third ripple effect is competition and market positioning. Wearables are in a new phase. Growth is still present, but the market is more contested and regionalized. Global shipment data shows a large, resilient wearables market with cyclical shifts among top brands. Smartwatch shipments are projected to return to growth, and vendors increasingly differentiate with health insights and AI coaching rather than raw hardware specs.
Apple’s advantage has long been credibility plus ecosystem integration. A credible “anxiety vs arrhythmia” experience reinforces both. It keeps Apple’s health story in the premium lane and makes the watch feel like a safety tool rather than a gadget. Competitors can copy sensors, but they struggle to copy regulatory relationships, clinical validation pipelines, and the trust that comes from conservative framing.
A snapshot of the market and regulatory environment around this shift:
| Area | What Is Changing | Why It Matters For “Anxiety Vs Arrhythmia” |
| Regulation (U.S.) | Clearer exemptions for low-risk wellness claims, scrutiny for disease claims | Incentivizes careful language and cautious “notification” framing. |
| Clinical adoption | More clinicians see wearable rhythm data routinely | Raises demand for standardized workflows and reduces tolerance for noisy alerts. |
| Consumer behavior | More self-monitoring, more expectation of answers | Increases pressure for triage experiences that reduce panic without overpromising. |
| Competitive differentiation | AI coaching and health insights become the main battleground | Rewards companies that can validate algorithms and design safer guidance. |
| Liability and trust | Users may treat signals as diagnoses | Forces manufacturers to emphasize limitations while still providing meaningful action steps. |
The “winners vs losers” lens clarifies who benefits if this is done well:
| Group | Likely Upside | Likely Risk If The Experience Is Poorly Designed |
| People at higher AFib risk | Earlier detection and better confirmation workflows | False reassurance if the watch feels too definitive |
| People prone to health anxiety | Fewer unnecessary alarms and clearer recheck steps | New obsession with stress metrics or constant checking |
| Clinicians | Better-quality patient-generated data | More messaging and interpretation if outputs remain ambiguous |
| Health systems | Fewer low-value visits and tests | Demand spikes if alerts are too frequent or confusing |
| Apple and competitors | Differentiation through “safer AI” health features | Regulatory backlash or trust erosion if marketed as diagnosis |
One subtle point deserves emphasis. The goal is not to stop people from seeking care. The goal is to align urgency with true risk. A well-designed classifier encourages immediate care when red flags appear, while preventing panic-driven overutilization when signals suggest low risk.
What Comes Next: The Milestones That Will Decide Whether This Helps Or Harms?
The future of “anxiety vs arrhythmia” will be decided by three kinds of evidence, not by launch-day hype.
First, accuracy evidence in real-world conditions. The key tests will not be conducted only in clean lab settings. They will measure performance when people are moving, sweating, stressed, and living normal lives. They will test how the watch behaves when the signal quality is poor and when symptoms are intense. This is where many consumer health algorithms fail, not because the model is weak, but because the edge cases dominate human experience.
Second, outcome evidence, not just detection evidence. A mature evaluation framework will ask:
- Does this reduce unnecessary emergency visits for palpitations?
- Does it reduce the number of low-value diagnostic cascades?
- Does it increase appropriate confirmatory testing when true arrhythmias occur?
- Does it lower anxiety and symptom preoccupation over time?
This is the hardest part because it requires measuring psychology, behavior, and utilization, not just rhythm classification.
Third, communication evidence. The future hinges on how the device speaks to people. The safest wearable health experiences do three things consistently.
They use calibrated language. They avoid absolute claims. They do not label mental health conditions. They describe signals as “consistent with” or “suggestive of,” then guide next steps.
They provide a structured pathway. Instead of dumping data, they offer a short decision tree: capture ECG, recheck stillness, track triggers, seek care if red flags, follow up non-urgently if persistent.
They respect that fear is part of the symptom. The device must reduce fear without dismissing it. That is a design problem as much as it is an algorithm problem.
If Apple pushes this forward, expect a strategy that looks more like coaching than diagnosis. The watch will likely expand the “recheck protocol” idea: guiding the user to sit still, record an ECG if possible, and repeat measurements over a short time window before escalating. It may also integrate palpitations into broader health dashboards that include sleep quality, recovery, and stress signals, aiming to contextualize episodes rather than isolate them as cardiac emergencies.
There are also regulatory milestones to watch in 2026.
One is how the FDA’s wellness-versus-medical boundary evolves in practice. If the agency becomes stricter about features that can be interpreted as disease detection, companies will respond by softening language and emphasizing general wellbeing. If the agency provides clearer safe harbors for certain types of coaching features, companies will move faster.
Another is how AI-enabled device update policies mature. Modern health algorithms improve over time. Regulators increasingly want visibility into what changes, how they are tested, and how safety is maintained. That will shape how quickly Apple and others can upgrade classifiers post-launch without re-running full review cycles.
A forward-looking view of possible scenarios:
| Scenario | What Apple And Rivals Would Do | What Users Would Experience |
| Conservative medical-adjacent lane | Keep “arrhythmia” claims narrow, expand guidance features cautiously | Fewer alerts, more structured recheck steps, strong emphasis on confirmation |
| Aggressive wellness lane | Frame stress and palpitations as “insights” and “coaching” | Faster feature rollout, more metrics, risk of users assuming medical certainty |
| Hybrid triage lane | Combine regulated AFib notifications with non-diagnostic anxiety-aware guidance | Best balance if designed well, but the messaging must be exceptionally clear |
| Backlash and retrenchment | Reduced claims after false-alarm controversies | Less innovation, more skepticism, slower adoption of wearable health tools |
Predictions should be labeled, and this is one: the most likely near-term outcome is the hybrid triage lane. Apple will maintain regulated heart features with conservative labeling, while building a richer “episode management” experience around palpitations that offers calming protocols, rechecks, and ECG prompts without claiming to diagnose anxiety. That approach aligns with both regulatory incentives and the need to reduce unnecessary alarm.
The broader implication is bigger than Apple. Wearables are evolving into always-on, always-available triage systems. In that world, the defining competitive advantage is not the sensor. It is the ability to translate uncertain physiological signals into safe, useful decisions. “Apple Watch Anxiety Vs Arrhythmia” is a headline for a deeper trend: technology companies are becoming intermediaries between symptoms and care pathways.
The public does not actually want more data. It wants clearer meaning. If this classifier reduces panic without hiding danger, it becomes a template for the next decade of digital health. If it overpromises certainty, it will deepen mistrust and make clinicians more defensive about patient-generated data. The difference will come down to evidence, language, and design discipline, not just machine learning.