At a White House press conference, President Donald Trump appeared alongside key health officials to unveil a set of policy changes focused on autism and maternal health. The administration stated that the Food and Drug Administration (FDA) would begin updating drug labels for acetaminophen — a widely used painkiller best known by the brand name Tylenol — to include warnings for pregnant women. The reasoning offered was that research has suggested a possible link between acetaminophen use during pregnancy and a higher chance of autism in children.
The announcement also included a surprising policy shift on leucovorin (folinic acid), a form of vitamin B traditionally used as a supplemental treatment in certain cancer therapies. Federal officials pledged to approve it as a therapy for children with autism symptoms, despite the fact that robust clinical evidence supporting this use is lacking. The administration further confirmed that state Medicaid programs, working with the Centers for Medicare and Medicaid Services (CMS), would begin covering this treatment.
Why the Announcement Was Controversial
The news created immediate backlash because the policies were introduced without clear or conclusive scientific evidence. Autism research remains complex, with genetic, environmental, and developmental factors all playing potential roles. No strong causal link between acetaminophen and autism has ever been proven, yet the administration presented the warning change as part of its broader push to address what it called the “root causes” of autism.
The press event highlighted rising autism rates in the United States, citing estimates that about 1 in 31 children are diagnosed with autism spectrum disorder today. Officials dismissed widely accepted explanations for the rise, such as expanded diagnostic criteria, better screening, and increased awareness. Instead, the administration suggested common medical practices — including frequent use of painkillers and vaccines — were partly responsible, despite extensive evidence disproving such claims.
Scientific Evidence on Acetaminophen
Studies over the past decade have explored whether there might be a connection between acetaminophen exposure during pregnancy and later developmental outcomes. For example, some cohort studies have reported that children born to women who reported frequent or long-term use of acetaminophen while pregnant had a slightly higher likelihood of being diagnosed with autism or ADHD.
However, large-scale controlled research has repeatedly shown that the evidence remains inconclusive. A 2025 Swedish sibling-controlled study involving nearly 2.5 million children found that when comparing siblings — where one was exposed to acetaminophen in the womb and the other was not — there was no meaningful increase in autism or ADHD diagnoses. This suggests that earlier associations may have been influenced by genetic and environmental confounding factors, rather than acetaminophen itself.
The FDA’s own review emphasized that while observational studies describe associations, there is no established causal mechanism. Acetaminophen has been one of the few pain and fever medications considered relatively safe for pregnancy, especially compared with ibuprofen or aspirin, which carry well-documented risks during gestation. Obstetricians also stress that untreated fever during pregnancy can lead to miscarriage, premature birth, or birth defects, making acetaminophen an important tool in maternal care.
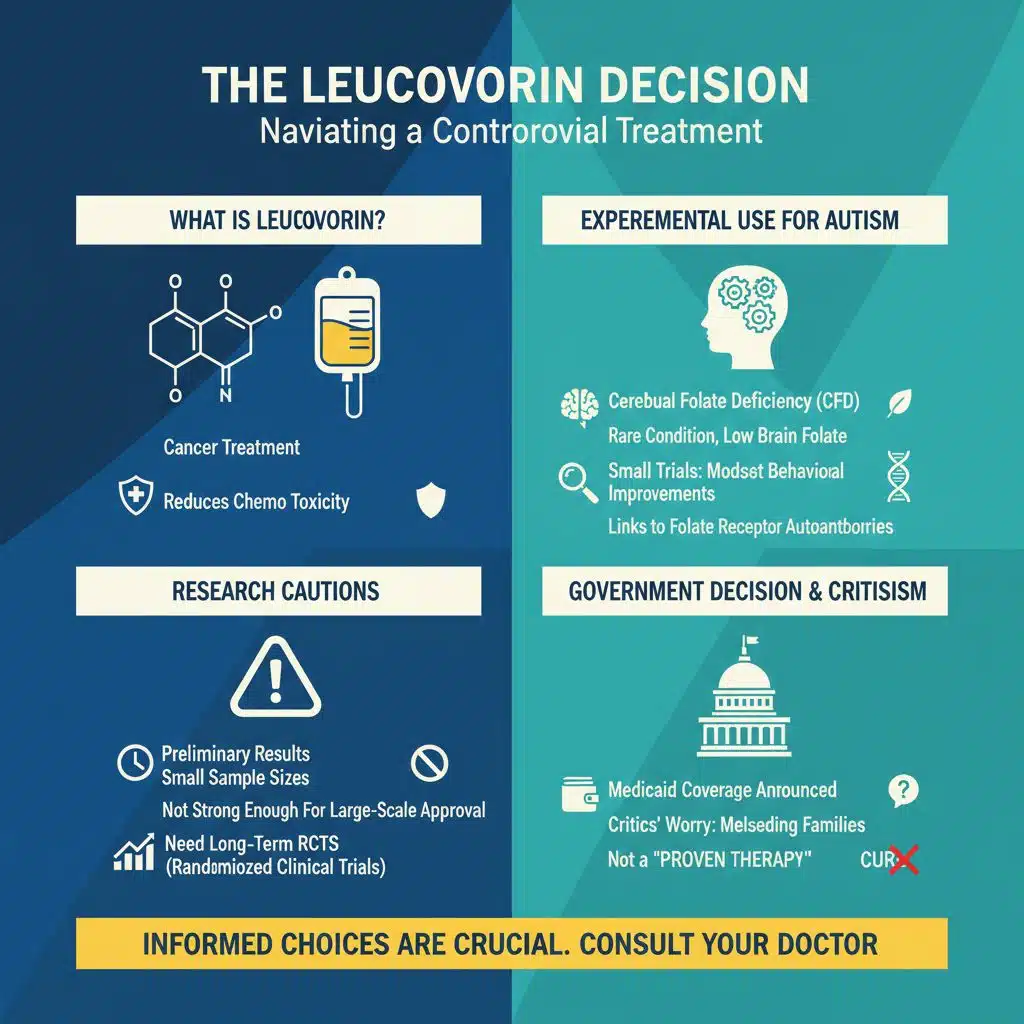
The Leucovorin Decision
Equally contentious was the government’s announcement that leucovorin would be recognized and reimbursed as a treatment for autism symptoms. In medical practice, leucovorin is usually given to cancer patients to reduce toxic effects of chemotherapy. More recently, small experimental trials have tested its use in children with cerebral folate deficiency (CFD), a rare condition where low folate levels in the brain can lead to autism-like symptoms.
These limited trials suggested modest behavioral improvements in some children, particularly those with specific folate receptor autoantibodies. Yet researchers caution that these results are preliminary, based on small samples, and not strong enough to justify large-scale approval. Major psychiatric organizations warn that more long-term, randomized clinical trials are needed before leucovorin can be reliably considered a treatment for autism.
Despite this, the administration said Medicaid would begin covering leucovorin for autism, a move that critics worry could mislead families into believing that the vitamin supplement is a proven therapy when it is not.
Reaction from the Medical Community
The announcement triggered strong opposition from professional medical bodies, autism advocacy organizations, and scientific experts. The American College of Obstetricians and Gynecologists (ACOG) stated that it is “deeply unsettling” for federal health agencies to issue new guidance that could affect millions of pregnant women without reliable data. They reiterated that acetaminophen, when used in moderation, remains the preferred option for managing pain and fever during pregnancy.
The Society for Maternal-Fetal Medicine also expressed concern. They emphasized that untreated fever itself can harm pregnancies and that discouraging acetaminophen could increase risks to both mothers and babies.
The American Psychiatric Association issued a statement opposing the use of leucovorin as an autism treatment at this stage, pointing out that decades of research have consistently shown no link between vaccines and autism, and warning that prematurely approving leucovorin risks misinforming families.
Autism advocacy groups, such as The Arc of the United States, said the announcement risks fueling stigma and distracting attention from what families truly need: support services, evidence-based therapies, and community resources.
Industry Response
The pharmaceutical industry quickly reacted as well. Kenvue, the company that manufactures Tylenol, strongly rejected the government’s claims, pointing to decades of safety data and multiple independent studies that show no proven connection between acetaminophen and autism. Vaccine manufacturers, including Merck, also spoke out, reiterating that extensive research over 25 years has found no evidence linking vaccines to autism.
Broader Context: Autism Rates and Diagnosis
Autism diagnoses have indeed risen in recent decades, but experts attribute the increase primarily to changes in diagnostic criteria, broader definitions, and heightened public awareness. For example, conditions that were once classified separately, such as Asperger’s syndrome, are now grouped under the broader autism spectrum. Increased screening in schools and pediatric practices has also identified more cases earlier in life.
Despite this consensus, the administration dismissed these explanations as insufficient, choosing instead to focus on widely used medications and vaccine schedules as contributing factors. This stance is in conflict with the positions of the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and other global health authorities.
Risks of the Policy Shift
Experts warn that the administration’s approach could have serious unintended consequences:
-
Pregnant women may stop using acetaminophen entirely, leaving fever and pain untreated — conditions that carry known risks for both mother and child.
-
Parents may pursue leucovorin treatments under the false impression that it is a validated therapy for autism, leading to misplaced hope and possible health risks.
-
Renewed public speculation linking vaccines to autism could undermine vaccination programs, lowering immunization rates and increasing the risk of outbreaks of diseases like measles and whooping cough.
The White House’s announcement represents a dramatic policy intervention in the ongoing debate over autism, pregnancy health, and safe medication use. By pledging to change labels on acetaminophen and expand leucovorin’s approval, the administration is moving ahead of the scientific consensus. While presented as a step toward protecting children, the policies have drawn sharp criticism from doctors, researchers, and advocacy groups who stress that the evidence is not yet strong enough to justify such sweeping changes.
As the FDA and CMS move forward, the situation will likely intensify debates over science, public health, and politics. Families affected by autism may find themselves caught between official government messaging, conflicting scientific evidence, and the urgent need for reliable, evidence-based care.