Have you ever wondered why your favorite local seafood joint is raising prices? Or why are the vibrant coral reefs you see in documentaries turning white? You are not the only one asking these questions. Many of us love the ocean but miss the invisible crisis happening right beneath the waves. This crisis is Ocean Acidification. Think of it as the “osteoporosis of the sea.”
Here is a startling fact. Since the Industrial Revolution, our oceans have become 30% more acidic. That number might sound small. But it is enough to dissolve the shells of tiny creatures that hold up the entire marine food web. I am going to walk you through exactly what is happening to our water. We will look at why it matters to your dinner plate and what we can do to fix it.
Stick around. We need to talk about how to keep our blue planet healthy for the kids growing up today.
What is Ocean Acidification?
Ocean acidification occurs when our seas absorb excess carbon dioxide from the atmosphere. It is a fundamental shift in ocean chemistry that turns life underwater upside down.
Definition and causes
Imagine the ocean as a global sponge. It constantly soaks up carbon dioxide (CO2) from the air. When cars, factories, and power plants burn fossil fuels like coal and oil, they pump massive amounts of CO2 into the sky.
Since 1750, humans have released over two trillion tons of this gas. The ocean absorbs about one-third of it.
When CO2 dissolves in seawater, it triggers a chemical reaction that lowers the water’s pH level. This makes the ocean more acidic. NOAA describes this as a rapid change that is happening faster now than at any time in the last 300 million years.
It is a direct threat to ocean health worldwide. Understanding this CO2 connection is the first step to seeing the bigger climate change picture.
The role of carbon dioxide (CO2) absorption
The ocean does us a huge favor by absorbing CO2. It acts as a buffer against climate change. But there is a limit to how much it can handle. Every year, we pump billions of tons of carbon into the air. Much of it ends up in the water.
When that extra CO2 mixes with H2O, it creates carbonic acid. This acid steals the carbonate ions that marine animals need to survive. It is like removing the bricks from a house while you are still trying to build it.
“The ocean breathes for us. What we do to the sea touches every part of life.” — Dr. Sylvia Earle
Just as too much sugar ruins a glass of lemonade, too much gas in the water harms the creatures that call it home.
Role of Fossil Fuel Emissions
Fossil fuel emissions are the engine driving this process. When we burn coal, oil, and gas, we send carbon dioxide straight into the atmosphere. Since the 1800s, industrial activity has pushed this cycle into overdrive. The oceans absorb nearly a third of that excess gas.
This rapid absorption changes ocean chemistry at a dangerous rate. The more we rely on fossil fuels for transportation and electricity, the harder it becomes for sensitive marine species to adapt. They simply cannot evolve fast enough to survive the shifting pH levels.
The Chemistry Behind Ocean Acidification
The chemistry here is straightforward but scary. As the ocean soaks up carbon dioxide, it becomes sour. This creates a hostile environment for life underwater.
Reduction in pH levels
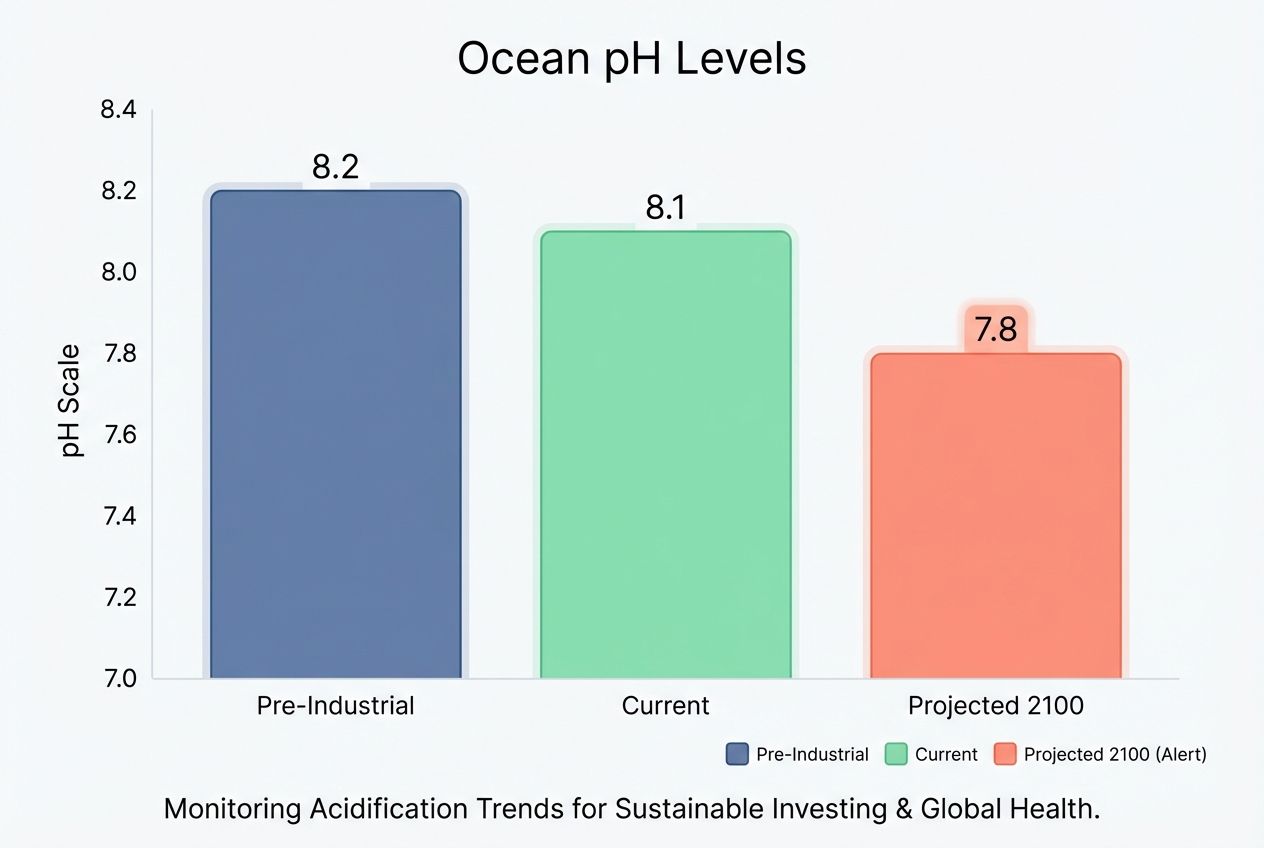
Scientists measure acidity using the pH scale. In pre-industrial times, the average ocean pH was about 8.2. Today, it has dropped to 8.1. That might look like a tiny difference. But the pH scale is logarithmic. This means a 0.1 drop actually represents a 30% increase in acidity.
To help you visualize this shift, here is how the pH change compares to other common substances:
| Substance | Approximate pH Level | Impact Context |
|---|---|---|
| Pre-Industrial Ocean | 8.2 | Healthy environment for shell growth. |
| Current Ocean | 8.1 | 30% more acidic. Shells begin to corrode. |
| Projected Ocean (2100) | 7.8 | 150% more acidic. Catastrophic for coral. |
| Pure Water | 7.0 | Neutral. |
Fish may not understand chemistry, but they feel this shift. It stresses their bodies and disrupts marine life everywhere. Even a small dip on the pH scale packs a massive punch.
Formation of carbonic acid and its effects
When carbon dioxide dissolves in seawater, it forms carbonic acid. This acid breaks apart and releases hydrogen ions. These ions are the real trouble. They bond with carbonate in the water. This leaves less carbonate available for animals like corals, oysters, and plankton.
These creatures need calcium carbonate to build their shells and skeletons. Without it, they struggle to grow. In severe cases, the acidic water actually starts to dissolve their existing shells.
Fish are also affected. In high-acid areas, some species lose their sense of smell. This confuses them and rattles food chains across fragile marine ecosystems.
Impacts on Marine Life
The impacts are visible right now. Tiny sea creatures are dissolving. Larger animals are confused. Chaos is spreading through ocean food chains.
Difficulty in shell and skeleton formation for calcifying organisms
Clams, oysters, crabs, and corals are the ocean’s architects. They need calcium carbonate to build their homes. As the ocean absorbs more carbon dioxide, that building material disappears.
This makes it incredibly hard for marine creatures to grow strong shells. In many cases, the shells grow thin and brittle.
This is not a theoretical problem. It is happening in the United States today. The Pacific Northwest is a hotspot for this issue.
- The Whiskey Creek Crisis: In the mid-2000s, oyster hatcheries in Oregon saw larvae dying by the millions. They discovered the acidic seawater was killing the babies before they could form shells.
- Dungeness Crabs: Recent NOAA studies show that young Dungeness crabs off the West Coast are showing signs of shell damage. This dissolves the sensory hairs they use to find food.
- Sea Butterflies: Tiny swimming snails called Pteropods are dissolving in the Southern Ocean. They are a critical food source for salmon and herring.
Healthy marine ecosystems depend on these tiny builders. Their struggle puts whole food chains at risk worldwide.
Disruption of coral reefs and marine ecosystems
Coral reefs are the rainforests of the sea. They are also the first victims of ocean chemistry changes. A drop in pH makes it nearly impossible for corals to build their calcium carbonate skeletons.
When coral breaks down, fish lose their homes. The reef becomes broken and bleak. A study found that parts of the Great Barrier Reef lost up to 50% of their coral cover in just three decades.
It is not just about the pretty corals. Food chains fall apart if the tiny creatures at the bottom cannot survive. Fish get fewer places to hide, which messes up life underwater and on shore.
Behavioral changes in fish and other marine species
Acidification messes with fish brains, too. As pH levels drop, certain fish start acting strangely. Some lose their survival instincts and swim toward danger instead of hiding.
Clownfish are a prime example. In acidic water, they cannot hear predators well. This means they get eaten more often. Predators like sharks also struggle. Acidification affects their sense of smell, making it harder to hunt. In 2012, scientists found that baby snapper in acidic water stopped fearing larger fish.
Squid and crabs can become more aggressive or lazy. These changes throw off natural food chains in marine ecosystems. If the prey acts weird, the predators leave. This forces species to migrate, disrupting the balance of the entire ocean.
Broader Effects on Humans and the Economy
You might think this is just a problem for fish. But ocean acidification hurts our wallets and our dinner plates. The ripple effects run deep.
Threats to global food security
Fish and shellfish feed billions of people. Ocean acidification puts this massive food source at risk. When acidic waters weaken shells, oysters, clams, and crabs die off. As their numbers drop, seafood supplies fall short. This drives up prices for families everywhere.
Many countries rely on fish as their main protein source. Small coastal communities depend on healthy marine ecosystems for food. If the food chain breaks at the bottom, there are fewer big fish at the top. This means empty nets for fishermen and uncertain meals for everyone else.
Economic losses in the fishing and tourism industries
Shrinking fish stocks hit fishing towns like a sledgehammer. Jobs dry up. Boats sit idle. Shops close down.
In the United States alone, the commercial fishing industry is massive. In 2022, it brought in over $4 billion. Ocean acidification puts this revenue at risk.
“The U.S. West Coast shellfish industry contributes over $270 million annually to the economy. Acidification is a direct threat to thousands of jobs in rural coastal areas.”
Tourism suffers too. People flock to places like the Florida Keys to see bright coral reefs. Dying reefs scare them away. Less healthy corals mean fewer visitors, which hurts hotels, dive shops, and guides.
Increased coastal flooding and erosion risks
Reefs are our first line of defense against storms. They break the waves before they hit the shore. Rising ocean acidification weakens these natural barriers. Without strong reefs, waves hit beaches harder. This washes away sand and soil much faster.
Climate change is already driving stronger storms. In 2022, NASA confirmed that coastal flooding is happening more often in the U.S. due to high tides and changing sea levels.
Communities are losing land. Cities spend billions repairing storm damage. We are facing a future where we have to choose between expensive sea walls or retreating from the coast entirely.
Potential Solutions to Ocean Acidification
The situation is serious. But hope is not lost. People are working hard to make the oceans healthier again.
Initiatives to Cut Carbon Emissions
The most effective solution is to stop the problem at the source. We must cut carbon emissions to slow ocean acidification. Here is how groups and governments are tackling it:
- Clean Energy Switch: Moving to wind and solar power reduces the need to burn coal. This keeps billions of tons of CO2 out of the air.
- Electrifying Transport: Every electric car on the road means fewer tailpipe emissions entering our skies and oceans.
- Reforestation: Planting trees acts as a natural brake. Forests soak up extra carbon dioxide before it hits the water.
- Carbon Pricing: Governments are setting limits on factory emissions. This forces companies to lower pollution or pay heavy fines.
- The Paris Agreement: Since 2015, 195 countries have united to keep global warming in check. This international teamwork is vital for ocean health.
Developments in Carbon Capture Technologies
Cutting emissions is step one. Step two is removing the carbon that is already there. New tech is emerging to fight this silent killer.
Direct Air Capture plants are essentially giant vacuums for CO2. As of 2024, over 25 of these plants are operating worldwide. They pull gas from the air and store it underground.
Another exciting method involves “coastal weathering.” Projects like Project Vesta are testing the use of crushed olivine sand on beaches. As waves hit the sand, it triggers a chemical reaction that safely locks away CO2 and lowers acidity locally. Large companies are investing billions in these solutions. The race is on to make them cheap enough to use everywhere.
Enforcement of Supportive Environmental Policies
Strong laws act as the guardrails for our planet. Governments are moving faster to protect marine health.
In the US, the Inflation Reduction Act provided massive funding for climate resilience and coastal protection. Strict limits on industrial waste keep toxic chemicals out of the water.
Protected zones act like sanctuaries. By stopping fishing and mining in specific coral reefs, we give the ecosystem a chance to recover. These “Blue Parks” allow biodiversity to bounce back.
City planners are also getting involved. They are designing defenses against the flooding and erosion linked to shifting ocean chemistry. It is a team effort, from the local mayor to the United Nations.
Ongoing Research and Predictive Insights
We cannot fix what we do not measure. Scientists are keeping a hawk-eye on ocean pH levels to predict what comes next.
Tracking Changes in Oceanic pH
To spot changes in ocean health, experts use advanced tech. They rely on sensors, satellites, and autonomous robots.
The Global Ocean Biogeochemistry Array uses “Argo floats.” These are robotic cylinders that drift through the sea, diving deep and surfacing to beam real-time data back to labs.
Over the last 200 years, pH levels have dropped from 8.2 to below 8.1. That is a massive shift for marine life. These records are our early warning system. They tell us which marine ecosystems are crashing first.
Future Impact Predictions on Marine Biodiversity
The forecasts are sobering. Scientists warn that ocean acidification could slash marine biodiversity by the year 2100. If trends continue, many creatures like oysters and corals will fail to grow shells. Fish will lose their survival instincts. We could see the collapse of entire reef systems.
By 2050, experts expect a sharp fall in fish stocks. Breeding grounds are disappearing. Young fish face too many challenges to reach adulthood.
This is not just about losing species. It is about losing the food security for the entire planet. Protecting marine ecosystems is the only way to ensure we have seafood in the future.
The Bottom Line
Our oceans are changing faster than we ever expected. They currently absorb 30 percent of the world’s carbon dioxide. This invisible gas from burning fossil fuels is lowering pH levels. It is causing chaos for marine ecosystems.
Creatures that need calcium carbonate are struggling to survive. Fish cannot adapt quickly enough. This hurts food chains and destroys local jobs. Coastal areas are losing their natural coral shields. This leads to more erosion and flooding. Since 1980, coral growth has dropped by up to 50 percent in some areas.
These shifts threaten millions of people who rely on the ocean for food and income. Every step we take to cut emissions matters. We need to act now for the sake of future generations.