On the global stage of medical innovation, Russia’s recent announcement of a cancer vaccine has sparked equal parts excitement and skepticism. This personalized mRNA-based cancer vaccine, designed to target cancer-specific mutations, claims to offer free treatment to patients by early 2025.
The bold promises of a vaccine tailored to individual tumors have intrigued the scientific community, marking a significant step forward in oncology research.
Cutting-edge mRNA technology and artificial intelligence (AI) at the heart of this vaccine’s promise signal a new frontier in personalized medicine. However, concerns about its efficacy, scalability, and transparency remain significant.
This article delves into the science behind Russia’s cancer vaccine, exploring its potential, the doubts surrounding it, and its global implications.
Understanding Cancer Vaccines
Unlike preventive vaccines that protect against infections like measles or HPV, cancer vaccines are therapeutic. They aim to treat cancer by stimulating the immune system to recognize and destroy tumor cells.
These vaccines target neoantigens, unique mutations in a tumor that are not present in normal cells. By identifying these markers, cancer vaccines help the immune system distinguish between healthy and cancerous tissue.
Cancer vaccines are not new, but advancements in immunology and genomics have brought us closer to creating effective therapies. Therapies like Sipuleucel-T (Provenge), approved for prostate cancer, demonstrate the potential of immunotherapy to revolutionize oncology.
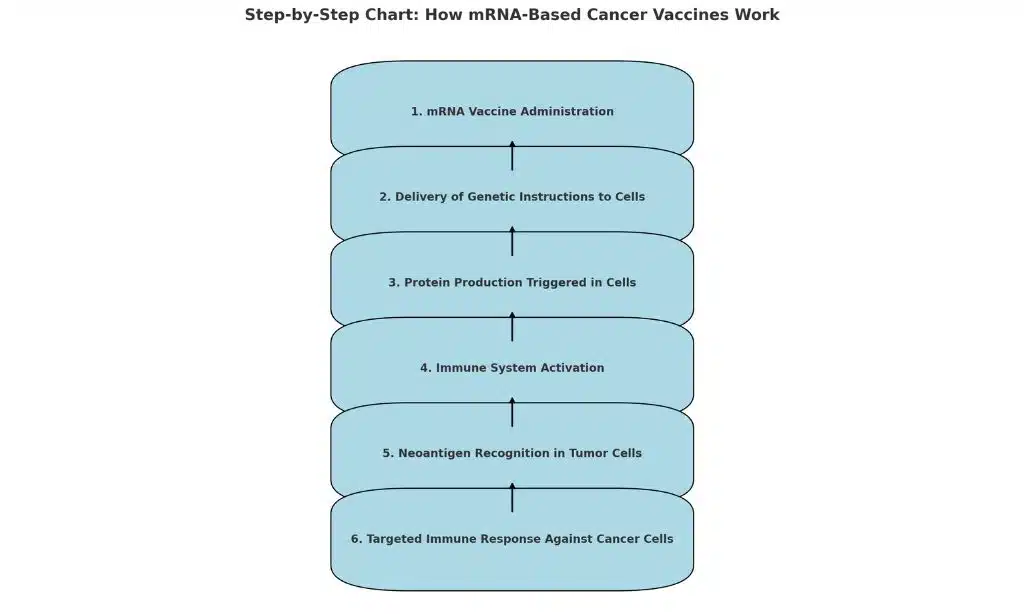
How mRNA-Based Cancer Vaccines Work
mRNA vaccines, popularized by COVID-19 vaccines like Pfizer-BioNTech and Moderna, deliver genetic instructions to cells, prompting them to produce proteins that trigger an immune response. In cancer treatment, mRNA vaccines encode for neoantigens specific to a patient’s tumor, enabling the immune system to recognize and attack cancer cells.
Why Personalized Cancer Vaccines Matter
Personalized cancer vaccines represent a major leap forward in oncology. Traditional treatments like chemotherapy often affect healthy cells and come with severe side effects. In contrast, personalized vaccines are designed for precision, targeting only cancerous cells. This approach not only minimizes side effects but also increases the likelihood of treatment success.
The Promises of Russia’s Cancer Vaccine
The Russian cancer vaccine, developed by the Gamaleya National Research Center, aims to revolutionize cancer treatment by tailoring therapies to individual patients. By analyzing the genetic profile of a tumor, scientists identify neoantigens to create a personalized vaccine. The goal is to activate the immune system to destroy tumor cells while preventing metastasis.
Pre-Clinical Success
According to Alexander Gintsburg, director of the Gamaleya Center, pre-clinical trials showed promising results. The vaccine reportedly suppressed tumor growth and reduced the risk of metastases. These findings, though encouraging, have yet to undergo peer review or be validated through large-scale human trials.
Integration of Artificial Intelligence
AI plays a pivotal role in the vaccine’s personalization process. By analyzing tumor genetic data, AI identifies neoantigens and designs the mRNA sequence, reducing production time to less than an hour. This integration not only accelerates vaccine development but also makes it scalable for wider use.
Free Accessibility for Patients
One of the most ambitious aspects of the project is Russia’s commitment to offering the vaccine free of charge. While this pledge has raised hopes, it also presents significant logistical and financial challenges.
Examples of mRNA Vaccine Success
Here are some notable examples of mRNA vaccine successes that highlight the potential of this technology:
COVID-19 Vaccines
- Pfizer-BioNTech (Comirnaty):
- The first mRNA vaccine to receive emergency use authorization during the COVID-19 pandemic.
- Demonstrated over 95% efficacy in preventing symptomatic COVID-19 in clinical trials.
- Successfully distributed to billions of people worldwide, proving the scalability of mRNA vaccine production.
- Moderna (Spikevax):
- Another highly effective mRNA vaccine for COVID-19, with similar efficacy rates to Pfizer-BioNTech.
- Pioneered advancements in cold-chain logistics for mRNA vaccine distribution.
Influenza mRNA Vaccine Trials
- Moderna’s Seasonal Flu Vaccine:
- Ongoing clinical trials for mRNA-based influenza vaccines aim to improve upon the low efficacy (40-60%) of traditional flu vaccines.
- Early results have shown strong immune responses and better adaptability to emerging flu strains.
Personalized Cancer Vaccine Success
- BioNTech’s Cancer Vaccine Trials:
- BioNTech has developed mRNA cancer vaccines targeting neoantigens, showing promising results in melanoma and other cancers.
- A recent study demonstrated that combining mRNA cancer vaccines with immune checkpoint inhibitors (like pembrolizumab) improved survival rates in advanced melanoma patients.
- Moderna’s Cancer Vaccine Partnership:
- Moderna, in collaboration with Merck, has launched mRNA-based cancer vaccines tailored to individual tumor profiles, showing positive responses in Phase 1 trials.
Rabies Vaccine
- CureVac has developed an mRNA-based rabies vaccine that showed strong neutralizing antibody responses in pre-clinical trials.
- This vaccine could significantly improve accessibility and effectiveness in regions with limited healthcare infrastructure.
Zika Virus Vaccine
- Moderna is developing an mRNA vaccine for Zika virus, targeting a neglected tropical disease with significant health risks in developing countries.
- Early-phase trials showed promising immune responses, setting the stage for further development.
HIV mRNA Vaccine Research
- Both Moderna and BioNTech are actively pursuing mRNA-based HIV vaccines.
- The vaccines aim to induce broadly neutralizing antibodies (bnAbs) against HIV—a long-standing challenge in traditional vaccine development.
Malaria Vaccine Development
- BioNTech has announced plans for an mRNA-based malaria vaccine, with clinical trials expected to begin soon.
- This vaccine aims to target malaria’s life-threatening stages with improved precision and efficacy compared to traditional approaches.
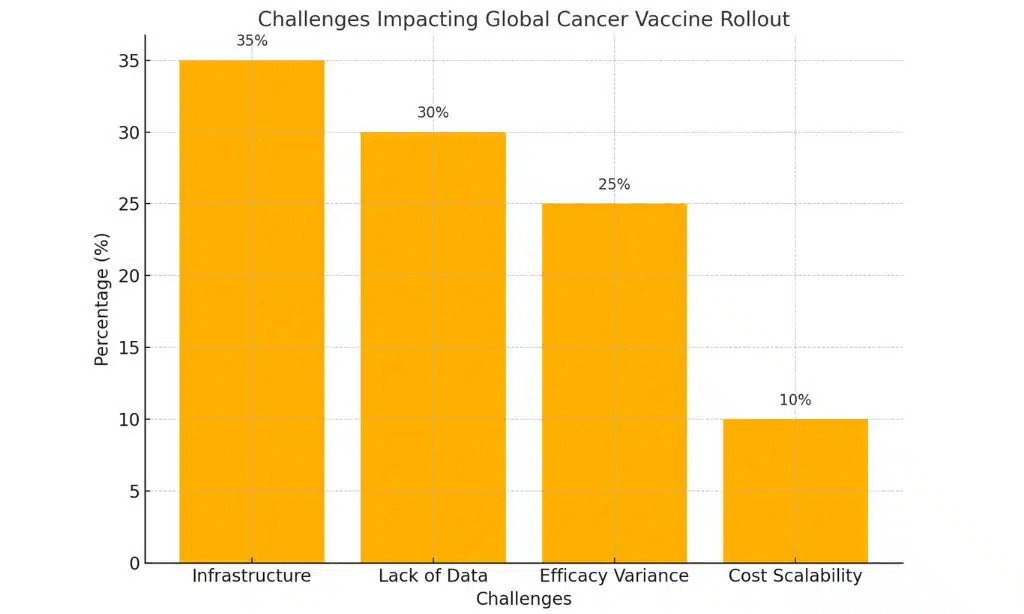
The Skepticism: Scientific, Ethical, and Logistical Concerns
| Concern | Description |
|---|---|
| Lack of Human Trial Data | Pre-clinical trials are promising, but human trial results are not yet published. |
| Efficacy Across Cancer Types | The vaccine may not be effective for all types of tumors due to genetic diversity. |
| Immune Response Challenges | Variability in immune system responses adds uncertainty to the treatment outcome. |
Ethical Considerations
- Transparency and Trust: Russian scientific institutions have faced criticism for a lack of transparency in past medical innovations, such as the Sputnik V COVID-19 vaccine.
- Free Distribution Feasibility: Providing personalized vaccines for free raises questions about funding, production scalability, and equitable access.
Logistical Hurdles
| Region | Existing Infrastructure Readiness | Key Challenges |
| North America | High | Distribution in rural areas |
| Europe | Moderate | Standardization of personalized vaccine protocols |
| Asia | Low | Limited access to AI-powered labs |
| Africa | Very Low | Lack of healthcare infrastructure and cold chains |
Historical Perspective on Cancer Vaccines
Cancer vaccines have a long and fascinating history, tracing back to early immunotherapy trials in the 20th century. These early efforts laid the foundation for modern advancements in targeting neoantigens and the revolutionary use of mRNA technology.
By understanding this timeline, we can appreciate how far the field has come and the immense potential it holds for the future of personalized medicine.
Early Immunotherapy Trials
The history of cancer vaccines dates back to the early 20th century, when pioneering immunologists began experimenting with therapies designed to boost the immune system’s ability to fight cancer. One of the earliest breakthroughs occurred in 1891, when Dr. William Coley used bacterial toxins, known as “Coley’s toxins,” to stimulate the immune response in cancer patients.
While rudimentary, this marked the beginning of cancer immunotherapy. Later, the development of Bacillus Calmette-Guerin (BCG) therapy in the mid-20th century advanced the field further, with BCG still being used as an effective treatment for bladder cancer today. These early trials laid the groundwork for modern approaches like neoantigen-targeted vaccines and checkpoint inhibitors, which have revolutionized cancer treatment.
By understanding the successes and limitations of these initial efforts, researchers have been able to refine and develop more sophisticated immunotherapies.
Breakthroughs in Neoantigen Targeting
The development of neoantigen-based therapies represents a significant leap forward in personalized medicine. Neoantigens, derived from mutations unique to individual tumors, were first identified as a potential therapeutic target in the early 2000s. Initial studies demonstrated that these tumor-specific markers could be used to stimulate an immune response, leading to tumor destruction without harming healthy cells.
By the 2010s, advancements in genomic sequencing and bioinformatics enabled scientists to identify these neoantigens with greater precision, paving the way for vaccines tailored to each patient’s unique tumor profile. Companies like BioNTech and Moderna leveraged these advancements to develop mRNA-based vaccines capable of encoding neoantigens, showcasing promising results in pre-clinical and early-phase trials.
These breakthroughs have positioned neoantigen-targeting therapies as a cornerstone of the next generation of cancer immunotherapy, with the potential to dramatically improve outcomes for patients with aggressive or treatment-resistant cancers.
The Rise of mRNA Technology
The emergence of mRNA vaccines has revolutionized oncology by offering a groundbreaking approach to treating cancer. Initially developed for infectious diseases, mRNA technology gained prominence with the success of COVID-19 vaccines like Pfizer-BioNTech and Moderna. The same principles—delivering genetic instructions to produce antigens—are now being applied to target neoantigens in cancer cells.
Unlike traditional cancer therapies, mRNA-based treatments are highly adaptable, enabling rapid personalization based on a patient’s unique tumor profile. This adaptability, combined with advancements in bioinformatics and genomic sequencing, has propelled mRNA technology to the forefront of cancer research.
Clinical trials for mRNA-based cancer vaccines are showing promising results, with improved immune responses and fewer side effects compared to conventional therapies. By leveraging the body’s own cellular machinery, mRNA technology is paving the way for a new era of precision medicine in oncology.
Global Implications of Russia’s Cancer Vaccine
Russia’s vaccine highlights the growing importance of personalized medicine in oncology. If successful, it could shift global research priorities toward neoantigen-based therapies, encouraging further investment in mRNA technology.
Impact on Healthcare System
A successful rollout of Russia’s cancer vaccine could profoundly influence global healthcare systems by shifting priorities toward personalized medicine. Such an initiative could redefine resource allocation, emphasizing the need for genomic analysis facilities, AI-driven diagnostics, and advanced vaccine distribution networks.
Additionally, the free distribution model proposed by Russia might inspire other nations to adopt similar strategies, potentially making personalized treatments more accessible and equitable. This could lead to a restructured healthcare economy, prioritizing prevention and precision treatment over generalized therapeutic approaches, ultimately transforming how national health policies are crafted worldwide.
Competition in the Global Vaccine Race
Countries like the US and Germany are also developing mRNA cancer vaccines. Russia’s announcement could spur competition or collaboration, accelerating innovation. Companies such as BioNTech are already conducting clinical trials for similar vaccines, thereby increasing the stakes in this scientific competition.
Implications for Developing Countries
A free, scalable cancer vaccine has the potential to revolutionize cancer treatment in low-income nations. However, concerns about affordability, distribution, and political factors may limit its global reach.
Broader Applications of mRNA Technology
The success of Russia’s vaccine could pave the way for mRNA-based treatments for other diseases, including autoimmune disorders and infectious diseases. AI integration could further accelerate advancements in personalized medicine.
Economic and Political Implications
Russia’s cancer vaccine initiative could significantly bolster its global standing in medical innovation. By pioneering a personalized mRNA-based approach to cancer treatment, Russia is positioning itself as a leader in cutting-edge medical research.
If successful, this vaccine could showcase the country’s ability to address one of humanity’s most pressing health challenges, potentially elevating its reputation in the scientific and healthcare communities. Additionally, the free accessibility promise underscores a public health commitment that may set a precedent for other nations, further solidifying Russia’s influence in the global medical arena.
However, transparency and collaboration will be key to gaining the trust of the international community and translating this innovation into a sustainable legacy.
Comparison Between Sputnik V and the Cancer Vaccine
| Aspect | Sputnik V (COVID-19 Vaccine) | Cancer Vaccine |
|---|---|---|
| Purpose | Prevent COVID-19 by inducing immunity | Treat cancer by targeting neoantigens |
| Technology | Adenoviral vector technology | mRNA-based personalized therapy |
| Development Timeline | Emergency use authorization in less than a year | Pre-clinical trials completed; human trials pending |
| Global Distribution | Widely distributed to over 70 countries | Planned free domestic rollout; global access TBD |
| Skepticism and Trust | Faced transparency concerns and data delays | Similar concerns regarding transparency |
| Innovation | Accelerated vaccine development using existing platforms | Integration of AI for rapid vaccine tailoring |
| Public Perception | Mixed due to geopolitical influences | Public cautiously optimistic; skepticism remains |
Ethical Dimensions of Personalized Medicine
The use of genetic data in vaccine development raises significant privacy concerns that must be addressed to ensure public trust and ethical practices. Collecting and storing genomic information, which is highly sensitive and unique to each individual, creates risks of unauthorized access, misuse, or even discrimination based on genetic predispositions.
Additionally, there is a pressing need to establish robust data protection frameworks to govern how this information is handled. Transparent policies, anonymization of genetic data, and strict regulatory oversight are crucial to mitigate these concerns.
Furthermore, clear communication with patients about how their data will be used, stored, and shared can help alleviate fears and encourage participation in personalized vaccine programs.
Takeaways
Russia’s cancer vaccine represents a bold step forward in personalized medicine, combining cutting-edge mRNA technology with AI-driven innovation. While its promises are immense, so are the challenges. As the world watches, the vaccine’s success will depend on rigorous testing, global collaboration, and transparency.
If successful, this vaccine could revolutionize oncology, offering hope to millions of patients worldwide. However, until more data is available, cautious optimism remains the best approach to understanding the science, skepticism, and global implications of this ambitious endeavor.