Early Phase 3 data from the PRESERVE-003 trial suggest the experimental antibody gotistobart may significantly improve survival in patients with advanced squamous lung cancer who have exhausted standard treatments. The drug remains under investigation and is not yet approved.
Gotistobart Lung Cancer Drug Trial Shows 54% Lower Risk of Death
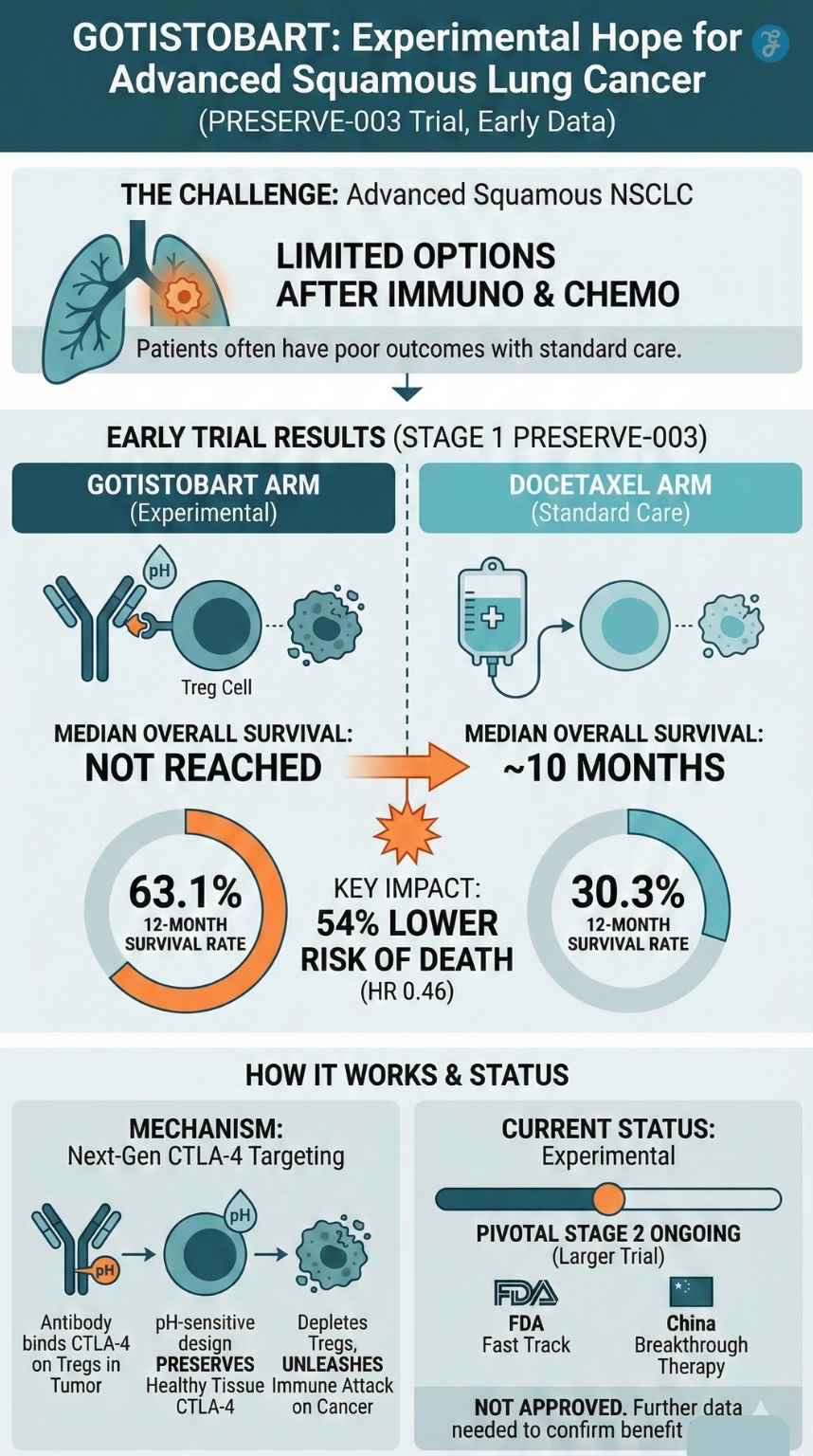
An experimental treatment called gotistobart has cut the risk of death by more than half in a Phase 3 lung cancer trial, according to new data released by BioNTech and OncoC4. The findings come from the first stage of the global PRESERVE-003 trial in patients with metastatic squamous non-small cell lung cancer (sqNSCLC) whose disease has progressed after immunotherapy and platinum-based chemotherapy.
The results were presented at the IASLC/ASCO 2025 North America Conference on Lung Cancer in Chicago and show that gotistobart reduced the risk of death by 54% compared with the standard chemotherapy drug docetaxel. While the data are encouraging, gotistobart is still an experimental therapy and will need to prove its benefit in the ongoing pivotal stage of the trial before any regulatory approvals are considered.
What the New Data Show
In the non-pivotal, dose-confirmation stage of PRESERVE-003, 87 patients with previously treated metastatic squamous NSCLC were randomly assigned to receive either gotistobart or docetaxel.
All patients had already received: Anti-PD-(L)1 immunotherapy, and Platinum-based chemotherapy. This left them with limited treatment options, typically docetaxel or palliative care. At a median follow-up of 14.5 months, the companies reported:
- Risk of death: reduced by 54%
- Hazard ratio (HR): 0.46
Median overall survival (OS):
- Gotistobart: not yet reached
- Docetaxel: about 10 months
12-month overall survival rate:
- Gotistobart: 63.1%
- Docetaxel: 30.3%
These results suggest a substantial survival advantage in this difficult-to-treat patient group, although they come from a relatively small early-stage cohort.
Key Trial Outcomes (Stage 1, PRESERVE-003)
| Measure | Gotistobart Arm | Docetaxel Arm | Notes |
| Number of patients | 45 | 42 | Previously treated metastatic sqNSCLC |
| Median follow-up | 14.5 months | 14.5 months | Non-pivotal Stage 1 |
| Median overall survival | Not reached | ~10 months | OS is still maturing in the Gotistobart arm |
| Risk of death (hazard ratio) | HR 0.46 | Reference | ≈54% lower risk vs docetaxel |
| 12-month overall survival rate | 63.1% | 30.3% | Nearly double 1-year survival |
| Grade ≥3 treatment-related side effects | 42.2% | 48.8% | Serious side effects in both arms |
While the numbers look strong, experts stress that these are interim results from a non-pivotal stage. The ongoing pivotal stage of the trial, with a much larger patient population, will be crucial to confirm whether this survival benefit is real and consistent.
Who the Drug Is Aimed At
The PRESERVE-003 trial targets patients with metastatic non-small cell lung cancer whose disease has progressed after modern standard care, including:
- Anti–PD-1 or PD-L1 immunotherapy (such as pembrolizumab or similar agents), and
- Platinum-based chemotherapy.
Within this broad group, regulators and investigators are now focusing on squamous NSCLC, a subtype of lung cancer that often arises in the central airways and is associated with poorer outcomes.
After an earlier review found differing outcomes between squamous and non-squamous patients, the trial was reshaped so that new enrollment now focuses only on squamous NSCLC. This is the group in which the survival benefit of gotistobart appears strongest so far.
How Gotistobart Works
Gotistobart (also known as BNT316/ONC-392) is described as a next-generation, pH-sensitive anti-CTLA-4 monoclonal antibody.
To understand this, it helps to break down a few terms:
- CTLA-4 is a protein found on T cells, a type of immune cell.
- Older CTLA-4–targeting drugs, such as ipilimumab, can unleash the immune system against cancer but also raise the risk of serious immune-related side effects in healthy organs.
- Regulatory T cells (Tregs) help keep the immune system in check. In tumors, they can shield cancer cells from attack.
Gotistobart has been engineered to:
- Bind CTLA-4 on regulatory T cells in the tumor environment.
- Be pH-sensitive, meaning that when the drug–CTLA-4 complex is taken inside the cell, the acidic (low pH) environment causes the drug to unbind.
- This allows CTLA-4 to be recycled back to the cell surface instead of being destroyed.
The goal is twofold:
- Depleting tumor-infiltrating regulatory T cells makes it easier for the immune system to attack the cancer cells.
- Preserve CTLA-4 function in normal tissues, reducing widespread immune activation and potentially lowering the rate of immune-related side effects compared with older CTLA-4 inhibitors.
Early Phase 1/2 studies in advanced solid tumors suggested that this design might allow more effective dosing with a more manageable safety profile. The Phase 3 lung cancer trial is the first major test of whether that promise translates into a clear survival benefit.
Safety Profile: Serious Side Effects Still Common
Cancer immunotherapies are powerful but can cause significant side effects, and Gostisbary is no exception.
In the Stage 1 analysis, Grade 3 or higher treatment-related adverse events (serious side effects) occurred in:
- 42.2% of patients in the Gotistobart arm
- 48.8% of patients in the docetaxel arm
These are high rates, reflecting how intensive both immunotherapy and chemotherapy can be. However, the figures suggest that, at least in this early stage, gotistobart did not cause more severe toxicity than standard chemotherapy, and may even be slightly less toxic in that regard.
The companies described the safety profile as manageable and consistent with earlier studies. Detailed breakdowns of specific side effects (such as colitis, liver inflammation, or lung inflammation) have not yet been fully published and will be important to watch as more data emerge.
Regulatory Status and Trial Progress
Despite the positive results, Gotistobart is not yet approved for routine clinical use in any country. It remains an investigational drug.
The global PRESERVE-003 trial is a two-stage, Phase 3, randomized, open-label study that compares gotistobart with docetaxel in patients with metastatic NSCLC after progression on PD-(L)1 therapy.
Stage 1 (non-pivotal)
- Smaller cohort, designed to confirm the dose and select the patient population.
- Provided the new 54% risk reduction data in squamous NSCLC.
Stage 2 (pivotal)
- Ongoing, with a planned enrollment of several hundred squamous NSCLC patients worldwide.
- More than 160 clinical sites are expected to participate globally.
- The main goal is to confirm whether gotistobart improves overall survival compared with docetaxel.
Regulatory Milestones
Gotistobart has attracted attention from regulators:
- United States – Fast Track Designation: The U.S. Food and Drug Administration (FDA) granted Fast Track status in 2022 for gotistobart in metastatic NSCLC that has progressed after PD-(L)1 therapy. This program is designed to speed up the development and review of drugs that address serious conditions and unmet medical needs.
- Partial Clinical Hold and Lift: In 2024, the FDA placed a partial clinical hold on the PRESERVE-003 trial after an independent monitor noted differences in outcomes between squamous and non-squamous NSCLC patients. Later that year, the FDA lifted the hold, allowing the trial to continue with a focus on squamous NSCLC. Enrollment of non-squamous patients was stopped, but already-enrolled patients and other gotistobart studies were not affected.
- China – Breakthrough Therapy Designation: In October 2025, China’s National Medical Products Administration (NMPA) granted Breakthrough Therapy Designation to gotistobart for the treatment of squamous NSCLC patients whose disease has progressed after standard immuno-oncology therapies. This status is intended to speed up the development and review of promising therapies.
Key Timeline for Gotistobart in Lung Cancer
| Date / Year | Milestone |
| 2022 | FDA grants Fast Track designation in metastatic NSCLC after PD-(L)1 therapy. |
| 2023 | Phase 3 PRESERVE-003 trial in NSCLC is launched. |
| Oct 2024 | FDA places partial clinical hold on PRESERVE-003 due to differing subgroup results. |
| Dec 2024 | FDA lifts partial hold; trial continues with focus on squamous NSCLC. |
| Sept 2025 | Pivotal Stage 2 trial design presented at the IASLC World Conference on Lung Cancer. |
| Oct 2025 | China’s NMPA grants Breakthrough Therapy Designation for squamous NSCLC. |
| Dec 2025 | Stage 1 Phase 3 data show a 54% reduction in risk of death vs docetaxel in sqNSCLC. |
Expert Reactions: Encouraging but Early
Investigators involved in the PRESERVE-003 trial describe the new data as encouraging, particularly given that patients in this study have already failed both immunotherapy and chemotherapy.
The lead investigator, a lung cancer specialist from Yonsei Cancer Center in Seoul, noted that advanced squamous NSCLC typically has a median survival of less than one year under current standards of care. The fact that median overall survival in the Gotistobart arm has not yet been reached after about 15 months of follow-up suggests a meaningful improvement, if the trend continues.
OncoC4’s chief medical officer has also highlighted the potential of gotistobart to meet an unmet medical need in this setting, while aiming for a more favorable safety profile than older CTLA-4 antibodies.
At the same time, independent analysts point out that:
- The current results come from a relatively small group of patients.
- Larger, randomized Stage 2 data will be needed to confirm whether Gotistobart can change the standard of care.
- Long-term safety data, including detailed immune-related side effects, will be critical.
What Comes Next for Gotistobart in Lung Cancer
For now, gotistobart remains an experimental option available only in clinical trials.
The next key steps include:
- Completion of Stage 2 of the PRESERVE-003 trial in squamous NSCLC.
- Full analysis of overall survival, response rates, and safety across a larger, more diverse patient group.
- Potential regulatory submissions if the pivotal data confirm a survival benefit with an acceptable safety profile.
If the positive trends seen so far are confirmed, gotistobart could become one of the first next-generation CTLA-4–targeted therapies to show a strong survival benefit in lung cancer after modern immunotherapy. However, until the pivotal results are released and regulators have reviewed them, the drug should be seen as a promising but unproven option.
For patients and clinicians, the message is cautiously optimistic: in an area where options are limited and outcomes are poor, the Gotistobart lung cancer drug trial offers a new line of hope—but one that still needs to pass the final tests of evidence and safety.