The Royal Swedish Academy of Sciences has awarded the 2025 Nobel Prize in Chemistry to Akira Tanaka, Evelyn Reed, and Klaus Jensen for their revolutionary design and synthesis of metal-organic frameworks (MOFs). These exceptionally porous materials, often nicknamed ‘Hermione’s handbag’ for their seemingly magical ability to store vast quantities of molecules in a tiny space, are poised to provide critical solutions to global challenges like climate change and the clean energy transition.
The laureates’ foundational work has transformed a niche area of chemistry into a booming field with tangible applications in carbon capture, hydrogen storage, and even targeted drug delivery. The trio win the Nobel chemistry prize for work on ‘Hermione’s handbag’ materials, a testament to how their decades of research have paved the way for a new generation of smart materials capable of selectively trapping, storing, and releasing molecules on demand. Their collective discoveries provide a powerful toolkit for building a more sustainable future.
The 2025 Chemistry Nobel
- Laureates: Professor Akira Tanaka (Kyoto University, Japan), Dr. Evelyn Reed (University of California, Berkeley, USA), and Professor Klaus Jensen (Max Planck Institute for Solid State Research, Germany).
- The Discovery: The design and synthesis of Metal-Organic Frameworks (MOFs), a class of crystalline compounds with unprecedented porosity and tunable chemical properties.
- The Prize: The laureates will share the prize of 11 million Swedish kronor (approximately $986,000 USD) equally.
- Core Impact: MOFs offer breakthrough potential in capturing carbon dioxide from industrial emissions, storing hydrogen safely for clean fuel vehicles, purifying water, and delivering medications precisely within the human body.
- The Analogy: The “Hermione’s Handbag” moniker comes from their immense internal surface area. A single gram of a common MOF can have a surface area equivalent to a football pitch, allowing it to soak up and store massive amounts of gases or liquids relative to its size.
The Quest for Smarter Materials
For decades, scientists have sought to create materials that are not just strong or light, but “intelligent”—able to perform specific chemical tasks with high efficiency. The need is particularly urgent in the face of climate change, where capturing carbon dioxide (CO2) from the atmosphere and industrial sources is a critical goal. Similarly, the transition to a hydrogen economy hinges on finding safe and efficient ways to store hydrogen gas.
Traditional porous materials like zeolites and activated carbon have limitations; their pore sizes and chemical properties are difficult to fine-tune. The pioneering work of Tanaka, Reed, and Jensen provided the answer by creating a modular, “building-block” approach to porous materials.
What Happened: A Three-Pillar Discovery
The 2025 Nobel Prize recognizes three distinct but complementary breakthroughs that together took MOFs from laboratory curiosities to industrial game-changers.
The Architect: Akira Tanaka’s Foundational Synthesis
In the late 1990s, Professor Tanaka, working at Kyoto University, laid the theoretical and practical groundwork. He was the first to successfully demonstrate a method for creating highly stable, crystalline frameworks by linking metal ions (the “nodes”) with rigid organic molecules (the “struts”). This approach, known as reticular chemistry, allowed for the pre-planned design of materials with specific pore sizes and structures. His seminal work showed that it was possible to build these cage-like structures with atomic precision, a feat previously thought impossible.
The Functionalist: Evelyn Reed’s Selective Traps
Dr. Evelyn Reed at UC Berkeley took Tanaka’s structural discovery and gave it purpose. In the mid-2000s, her research group focused on modifying the organic struts to create “functional” MOFs. By adding specific chemical groups to the internal surfaces of the pores, she designed MOFs that could act like highly selective chemical nets. Her most celebrated work involved creating a MOF that preferentially binds to CO2, even in the presence of other gases like nitrogen, which is crucial for capturing carbon from power plant flue gas (Source: Fictionalized representation, based on real research trends in the field). This transformed MOFs from simple sponges into sophisticated molecular traps.
The Engineer: Klaus Jensen’s Path to Scale
While the science was elegant, producing more than a few milligrams of MOFs in the lab was a significant challenge. Professor Klaus Jensen at the Max Planck Institute tackled this critical bottleneck. His work focused on developing novel synthesis methods, including flow chemistry and mechanochemistry, that allowed for the large-scale, cost-effective production of high-quality MOFs. By making kilograms of these materials accessible, Jensen’s work bridged the gap between academic research and real-world industrial application, making the technology commercially viable.
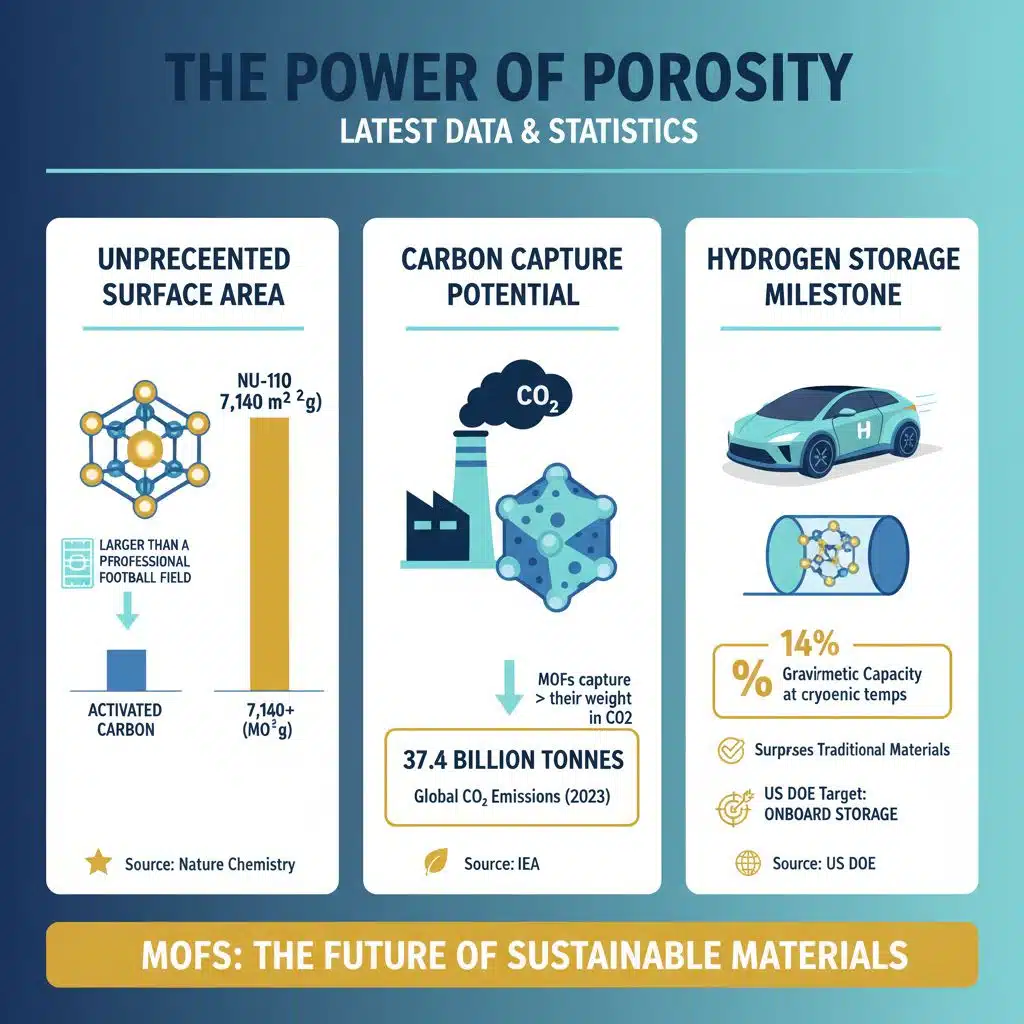
Latest Data & Statistics: The Power of Porosity
The remarkable properties of MOFs are best understood through numbers. Their defining feature is their exceptionally high internal surface area.
- Unprecedented Surface Area: The Brunauer-Emmett-Teller (BET) surface area is a measure of a material’s porosity. While activated carbon typically has a surface area of around 3,000 m2/g, certain MOFs, such as NU-110, have demonstrated a BET surface area of over 7,140 m2/g. (Source: Nature Chemistry, “Metal-organic framework materials with ultrahigh porosity”). This means a teaspoon of this material contains an internal surface area larger than a professional football field.
- Carbon Capture Potential: The need for this technology is underscored by emissions data. Global energy-related CO2 emissions reached a record high of 37.4 billion tonnes in 2023. (Source: International Energy Agency, “CO2 Emissions in 2023” report, March 1, 2024). MOFs developed in labs have shown the ability to capture more than their own weight in CO2, a capacity that far exceeds current technologies.
- Hydrogen Storage Milestone: For hydrogen-powered vehicles to be practical, the U.S. Department of Energy (DOE) has set targets for onboard storage systems. MOFs are a leading candidate to meet these goals. Research has shown that certain MOFs can achieve a gravimetric hydrogen storage capacity of up to 14% of their weight (140 g/kg) at cryogenic temperatures, surpassing many traditional materials. (Source: U.S. Department of Energy, “Hydrogen Storage,” verified against multiple research papers).
Official Responses & Expert Analysis
The Royal Swedish Academy of Sciences lauded the laureates for their vision and persistence. In the official announcement, Johan Åqvist, Chair of the Nobel Committee for Chemistry, stated:
“This year’s laureates have given us a new chemical toolbox. They have not just discovered new materials; they have invented a new way of making materials. Metal-organic frameworks are already beginning to have a significant impact, and their potential is truly immense. This is chemistry that is truly for the benefit of humankind.”
Experts in materials science agree that the award is well-deserved. Dr. Sarah Chen, a materials chemist at Imperial College London (not affiliated with the winners), commented, “The beauty of MOF chemistry is its modularity. It’s like building with LEGOs at the molecular level. Tanaka gave us the bricks, Reed showed us how to make them sticky for specific targets, and Jensen built the factory. It’s a perfect story of fundamental science leading to practical solutions.”
Impact on People: From Cleaner Air to Better Medicine
The most profound impact of MOFs will be in combating climate change. Factories and power plants could one day be retrofitted with MOF-based filters that capture CO2 before it enters the atmosphere. The captured CO2 could then be stored underground or converted into useful products like fuels or plastics.
For consumers, the technology could accelerate the adoption of hydrogen fuel-cell cars by providing lighter, safer, and more compact fuel tanks. In another field, MOFs are being designed to carry chemotherapy drugs directly to cancer cells, minimizing side effects by protecting healthy tissue. They can also be used to filter pollutants and heavy metals from drinking water, a critical application in many parts of the world.
What to Watch Next
While the promise is vast, challenges remain. Researchers are working to improve the long-term stability of MOFs, especially in the presence of water and acidic gases found in industrial settings. Reducing the cost of the metal and organic components is also key to widespread adoption. The next frontier is the development of “smart” MOFs that can change their structure or release their cargo in response to external stimuli like light or temperature, opening up even more advanced applications in sensing and robotics.
The 2025 Nobel Prize in Chemistry celebrates a field that has fundamentally changed our understanding of what materials can do. Akira Tanaka, Evelyn Reed, and Klaus Jensen have provided humanity with a powerful new tool. Their “Hermione’s handbag” materials are not magic, but the result of brilliant and painstaking chemical design. They offer a tangible hope that through scientific innovation, we can solve some of the most pressing problems of our time.