The video is just thirty seconds long, but it was enough to stop time for millions. A man named Bruno, paralyzed since 2018, stands at the bottom of a staircase and begins to climb, no exoskeleton, no robotics, just his own muscles firing. For the internet, it was a viral miracle; for the scientific community, it was a biological impossibility. But this isn’t magic.
This in-depth Polylaminin breakthrough analysis exposes the reality of a Brazilian therapy that is rewriting the rules of neuro-regeneration. Now, with a historic regulatory approval in January 2026, the “cure” is finally real. Still, a heartbreaking legal fine print has left millions of hopeful patients waiting on the other side of a locked door.
Key Takeaways
- The Science: Polylaminin creates a “molecular bridge” in the spinal cord, guiding neuron regrowth by mimicking the embryonic environment.
- The Results: In a pilot study, 100% of acute patients regained voluntary movement. Veterinary trials also showed walking recovery in chronically paralyzed dogs.
- The Catch: New 2026 trials are approved only for acute injuries (<72 hours), leaving chronic patients waiting for safety data.
- Global Context: Unlike Neuralink (chips) or stem cells, this is a unique structural biological repair developed entirely in Brazil.
- The Hurdle: Production requires harvesting human placentas, making global mass production a major logistical challenge.
Behind the Headlines: The 25-Year Quest
The story of Bruno’s recovery didn’t start on social media. It began twenty-five years ago in a quiet laboratory at the Federal University of Rio de Janeiro (UFRJ). Dr. Tatiana Coelho de Sampaio wasn’t looking for fame; she was hunting a single molecule called Polylaminin.
While the world cheered for the viral clip, a complex battle was unfolding in the halls of Brazil’s health regulator, ANVISA. The agency finally greenlit human clinical trials this month, legitimizing the science but creating a stark divide. The approval is strictly for acute injuries (less than 72 hours old), excluding the very chronic patients who made the video go viral. This is the inside story of that tension: the collision of a genuine scientific triumph with the desperate reality of those waiting for a cure.
This is the inside story of Polylaminin: the science, the struggle, and the complex reality behind the headlines.
The Ghost in the Machine
To understand why Polylaminin is revolutionary, we first have to understand why spinal cord injuries are so notoriously permanent.
The human body is generally excellent at healing. Break a bone, and it knits back together. Cut your skin, and it seals. But the Central Nervous System (CNS), the brain and spinal cord, is different. It is evolutionarily designed to protect itself from chaos.
When the spinal cord is severed, the body’s immediate reaction is panic. Inflammation floods the site. To prevent the damage from spreading, specialized cells called astrocytes rush in and form a dense, impenetrable wall known as a “glial scar.” This scar saves your life by sealing the breach, but it also seals your fate. It acts as a chemical and physical barrier. Any neuron that tries to grow across this gap hits the scar, becomes confused by hostile chemical signals, and eventually retracts or dies.
For decades, scientists threw everything at this wall. They tried stem cells to replace the dead neurons. They tried enzymes to dissolve the scar. They tried electrical implants to bridge the signal. While there have been incremental successes, nothing has consistently restored a robust biological connection in humans.
Enter Dr. Tatiana Coelho de Sampaio
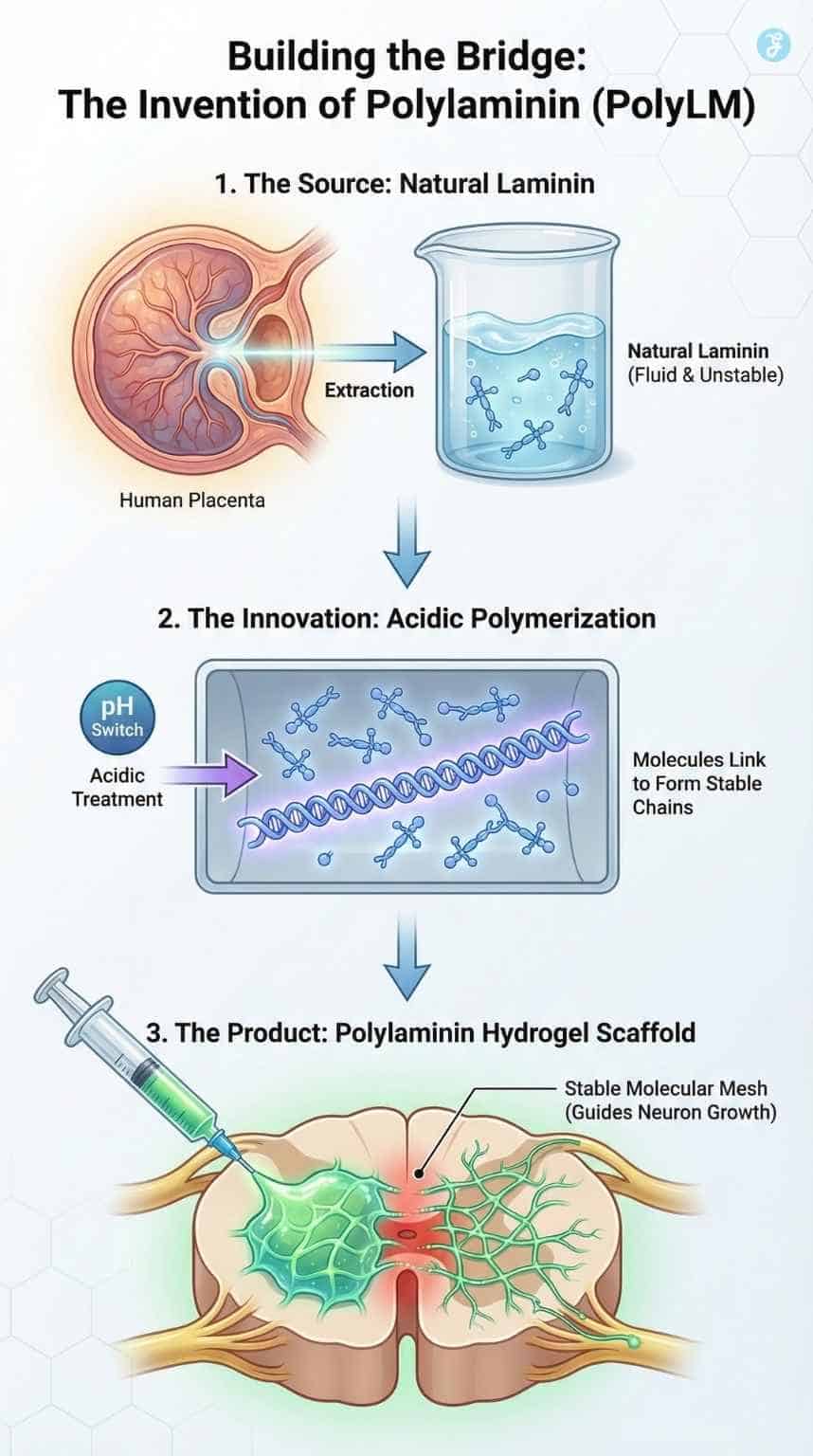
In the late 1990s, while much of the world was obsessed with the newly discovered potential of stem cells, Dr. Sampaio was looking at something simpler: the road, not the car. She focused on Laminin, a protein found abundantly in the human placenta and the developing embryo.
In the womb, Laminin is the “highway” that tells growing neurons where to go. It provides a sticky, comfortable track that encourages nerve fibers to extend. The problem? In adults, Laminin is fluid and unstable. If you inject it into a spinal injury, it washes away or degrades before it can do any good.
Dr. Sampaio’s “Eureka” moment wasn’t discovering the protein, but figuring out how to freeze it in time.
The Invention of Polylaminin
The breakthrough at UFRJ was a process of polymerization. By treating the natural Laminin molecule with a specific acidic process, the team found they could make the molecules link arms, forming long, stable chains. They called this super-molecule Polylaminin (PolyLM).
When injected into a spinal lesion, PolyLM doesn’t wash away. Instead, it self-assembles into a hydrogel—a stable, molecular mesh that mimics the environment of the embryonic brain.
The “Trojan Horse” Mechanism
Polylaminin works by tricking the body’s neurons. When a severed nerve fiber encounters this PolyLM mesh, it receives a chemical signal that overrides the “stop” signal of the glial scar. The neuron thinks, “I know this surface. This feels like the womb. It’s safe to grow here.”
The results in early testing were startling because PolyLM achieved three things simultaneously that other therapies could not:
- Neuroprotection: It reduced the massive inflammation that usually kills surviving neurons in the days after an accident.
- Angiogenesis: It promoted the growth of new blood vessels, ensuring the healing tissue had oxygen.
- Axonal Regeneration: Most importantly, it acted as a physical guide rail. Neurons grew through the lesion, reconnecting with the tissue on the other side.
This was not about putting new cells in (like stem cell therapy); it was about building a bridge so the patient’s own cells could cross the valley of death.
| Feature | Stem Cell Therapy | Polylaminin (PolyLM) |
| Primary Goal | Replace dead cells with new ones. | Repair the environment/structure. |
| Mechanism | Biological replacement. | Biomimetic scaffolding & guidance. |
| Main Challenge | Rejection, tumor formation, and uncontrolled growth. | Sourcing raw material (placenta), stability. |
| Status (Brazil) | Experimental/Various stages. | Phase 1/2 Clinical Trials Approved (2026). |
The Clinical Data [Fact vs. Hope]
Science is slow, but results speak loudly. The viral videos that surfaced in 2025 stem from a “compassionate use” pilot study conducted around 2018. This was not a formal, double-blind clinical trial, but a desperate measure for desperate cases. To separate the viral hype from the scientific reality, we must look at the specific data points generated by UFRJ. The evidence is divided into two distinct buckets: Animal (Chronic) and Human (Acute).
1. Animal Trials: The Chronic Hope
The strongest evidence that Polylaminin can treat old injuries comes from veterinary medicine. Dr. Sampaio’s team treated a cohort of dogs with chronic paraplegia, meaning they had been paralyzed for months or years before treatment.
- The Result: A significant number of these dogs regained the ability to walk.
- The Significance: This proves that the “glial scar” (the wall that blocks nerve growth) is not permanent. Polylaminin was able to remodel the scar tissue and guide neurons through it, even long after the initial injury. This is the scientific anchor for the hope held by chronic human patients.
2. Human Trials (Phase 1/Pilot): The Acute Reality
The medical team administered Polylaminin to eight patients. These were not minor injuries; they were classified as “complete” spinal cord transections. In standard trauma medicine, the prognosis for such patients is bleak. Spontaneous recovery of motor function occurs in roughly 15% of cases, and usually, it is minimal, perhaps a twitch of a toe or some return of sensation.
- Subjects: 8 patients with “complete” spinal cord injuries.
- Timing: Crucially, they were treated within 72 hours of their accident (Acute).
- The Outcome: Two patients died from accident-related trauma. Of the six survivors, 100% regained some voluntary motor control.
- Safety: The study confirmed the primary goal of Phase 1: the drug is safe. There were no adverse reactions like tumors or rejection, which are common risks in stem cell therapies.
3. The Crucial Caveat
It is vital to temper expectations. “Regeneration” does not mean a patient stands up and runs a marathon the next day.
- The Reality of Recovery: In both dogs and humans, the return of function was gradual. The nerves reconnected, but the muscles were atrophied, and the brain had “forgotten” how to walk.
- The Role of Rehab: The “miracle” required months of intense physical therapy to retrain the neural pathways. Polylaminin provided the hardware (the cable), but the patient still had to reinstall the software (the movement patterns) through hard work.
Bruno Drummond, the patient now famous on social media, was one of the survivors. He didn’t just regain sensation; he regained the ability to drive the muscles in his legs. The therapy didn’t make him “perfect”; rehabilitation was grueling and took years, but it gave him the biological hardware to work with.
“It changes the paradigm,” said Dr. Sampaio in a past interview with Brazilian media. “We aren’t just managing a disability. We are reversing the biological event of paralysis.”
The ANVISA Ruling [January 2026]
This brings us to the present day. On January 6, 2026, the Brazilian Health Regulatory Agency (ANVISA) issued the authorization that changed everything. They approved the Phase 1/2 Clinical Trial.
But the approval came with strict boundaries, designed to protect patients but destined to frustrate the public.
The Inclusion Criteria
The new trial is not open to everyone. It is ruthlessly specific:
- Injury Window: Must be treated within 72 hours of the accident.
- Injury Location: Thoracic region (T2 to T10 vertebrae).
- Age: Adults (18-72).
- Condition: Clinically stable.
Why Exclude Chronic Patients?
To the layman, this feels cruel. To the regulator, it is an ethical necessity. In an acute injury (fresh), the environment is chaotic but fluid. The scar hasn’t formed yet. Proving safety here is the first step. If you inject a new experimental substance into a chronic patient who has been stable for 10 years, and something goes wrong (e.g., infection, tumor, adverse reaction), you could take away the little independence they have left.
Furthermore, from a scientific data perspective, acute trials are “cleaner.” If you treat a fresh injury and the patient walks, you know it’s the drug. If you treat a chronic patient who has been doing rehab for 10 years, variables are messier.
ANVISA’s logic is: Prove it is safe in the fresh cases first. If that works, then, and only then, we open the door to the chronic millions.
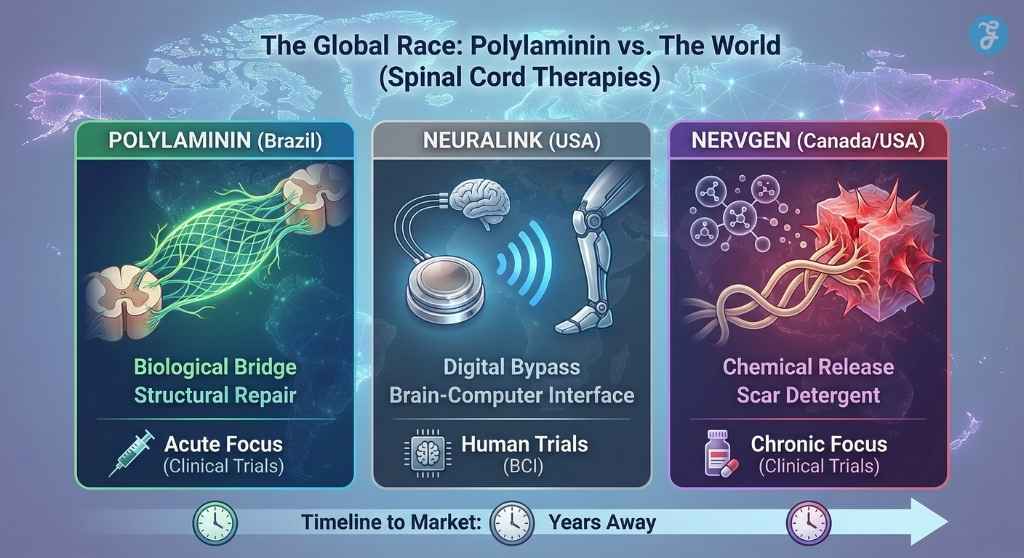
The Global Race: Polylaminin vs. The World
Brazil is not alone in the race to cure paralysis. To understand the significance of the ANVISA approval, one must look at the competition.
Currently, the primary rival to Polylaminin is NVG-291, a drug developed by the Canadian biotech firm NervGen. The two drugs represent opposing philosophies. NVG-291 acts as a “molecular detergent,” scrubbing away the chemical signals in the scar tissue that tell neurons to stop growing. Polylaminin, by contrast, acts as a “molecular bridge,” paving a new road over the damage.
The distinction is critical for patients. NervGen is currently testing its drug on chronic patients (those injured up to 10 years ago), aggressively targeting the market that Brazil’s regulators have deemed off-limits for now.
Meanwhile, in the United States, Neuralink (Elon Musk’s company) is pursuing a digital solution: bypassing the biological injury entirely with chips.
The Verdict: While Neuralink offers a technological workaround, and NervGen offers a chemical release, Polylaminin remains unique as a structural biological repair. It is the only therapy that attempts to physically rebuild the “highway” of the spinal cord using the body’s own embryonic blueprints.
How It Compares: At a Glance
To understand the uniqueness of Polylaminin, we must compare it to the two other heavyweights in the field: Elon Musk’s Neuralink and Northwestern University’s “Dancing Molecules.
| Feature | Polylaminin (Brazil) | Neuralink (USA) | “Dancing Molecules” (USA) |
| Approach | Biological Repair | Digital Bypass | Synthetic Mimicry |
| Mechanism | Uses natural protein (placenta) to bridge the gap. | Uses a microchip to transmit brain signals directly to devices/limbs. | Uses synthetic nanofibers to mimic proteins and signal cells. |
| Invasiveness | Low (Single Injection). | High (Brain Surgery/Implant). | Low (Single Injection). |
| Status | Phase 1/2 Clinical Trials (Acute). | Human Trials (BCI interface). | FDA Orphan Drug Designation (Preclinical/Early). |
| Advantage | Natural Integration: The body recognizes the protein. No batteries or wires. | Immediate Control: bypasses the injury entirely. | Scalability: Synthetic material is easier to mass-produce than the placenta. |
Why Brazil’s Approach is Unique
Neuralink is a “prosthetic” solution; it gives you a way to operate despite the injury. Polylaminin is a “restorative” solution; it attempts to fix the injury itself. Unlike the “Dancing Molecules,” which are synthetic, Polylaminin uses the exact biological material (Laminin) that nature uses to build the nervous system in the first place, potentially offering a more compatible “language” for our cells.
The “Valley of Death”: Manufacturing & Logistics
Why did it take 25 years? If the results are so good, why wasn’t this in hospitals a decade ago? The answer lies in the unglamorous reality of bio-manufacturing.
Polylaminin is not a synthetic chemical you can mix in a vat like Aspirin. It is a biological product. Its source material is the human placenta. To treat a single patient, you need a specific amount of purified Laminin. To treat thousands, you need a supply chain that rivals the Red Cross blood donation system.
- Collection: You need a network of maternity wards to collect placentas (which are usually medical waste).
- Screening: Every placenta must be tested for HIV, Hepatitis, and other pathogens.
- Extraction: The tissue must be processed to extract the Laminin protein.
- Polymerization: The proprietary process (held by UFRJ and Cristália) turns it into PolyLM.
- Sterilization: It must be made safe for injection into the spinal cord, a highly sensitive area.
For years, the project languished in what investors call the “Valley of Death”, the gap between a successful academic lab result and the massive funding needed to build a factory. The turning point was the partnership with Cristália, a Brazilian pharmaceutical giant known for its boldness in local innovation. Cristália bet on the project, investing millions to build the industrial process required to scale this up.
The Collision of Science vs. Hype
The Polylaminin Breakthrough saga is now entering its most dangerous phase: the collision of Science vs. Hype.
The “Dallas Buyers Club” Problem
With the viral success of Bruno’s story, Brazil is likely to see a wave of “judicialization” of health. Patients with chronic injuries may sue the state or the university, demanding access to the drug under “Right to Try” laws or compassionate use grounds. This places researchers in an impossible ethical bind: they want to help, but distributing the drug outside the trial could contaminate the data and get the entire project shut down by regulators.
The Global Spotlight
Until recently, this was a Brazilian story. Now, it is global. The U.S. FDA and European EMA are watching closely. If the Brazilian Phase 1/2 trial replicates the 100% success rate of the pilot study, we will see an international race to license the technology. This raises questions of biological sovereignty. The technology is Brazilian. The raw material (placentas) is Brazilian. Will the final drug be affordable for Brazilians, or will it be licensed to a global conglomerate and sold back at a premium?
The Manufacturing Bottleneck
Even if the trial is a success, the placenta supply chain is a limiting factor. Synthetic biology, creating Laminin in yeast or bacteria, is the next necessary hurdle. Research is ongoing, but currently, the “real thing” is still required for the polymerization to work correctly.
The Road Ahead [Timeline]
With the ANVISA approval in January 2026, the clock has officially started. Here is the realistic roadmap for what happens next:
- 2026-2027: Phase 1/2 Trials (The Current Step)
- Goal: Confirm safety and efficacy in a larger group of Acute patients (T2-T10 injuries within 72 hours).
- Constraint: This phase is strictly controlled. No chronic patients will be admitted yet. The data from this phase is required to prove that the injection doesn’t cause harm in a fresh injury.
- 2028+: Phase 3 Trials (The Big Hurdle)
- Goal: This is the massive, multicenter study required for final drug approval. It will involve hundreds of patients across different hospitals.
- The Funding Gap: This phase costs tens of millions of dollars. While UFRJ and Faperj (state funding) have carried the torch so far, global partners or massive government investment will be needed to fund a trial of this magnitude.
- The “Chronic” Question:
- If Phase 1/2 is successful in acute patients, the team will likely apply for a “breakthrough therapy” designation or a parallel trial for Chronic patients, citing the successful dog data. This is likely the earliest point (2-3 years from now) that chronic patients might legally access the drug in a trial setting.
Final Words: A Cautious Dawn
For twenty-five years, Dr. Tatiana Coelho de Sampaio worked quietly at UFRJ, fueled not by biotech millions but by public funding and a stubborn belief that the spinal cord can repair itself. Today, the secret is out, and the images of Bruno walking are indelible proof.
For chronic patients, the wait is painful, complicated by regulatory caution that currently restricts the drug to acute cases. Yet, the paradigm has shifted. This Polylaminin breakthrough proves the wait is no longer for a miracle that has already happened in the lab. The wait is now simply for the safety trials to catch up with the science. The bridge has been built; now, humanity must learn to cross it safely.