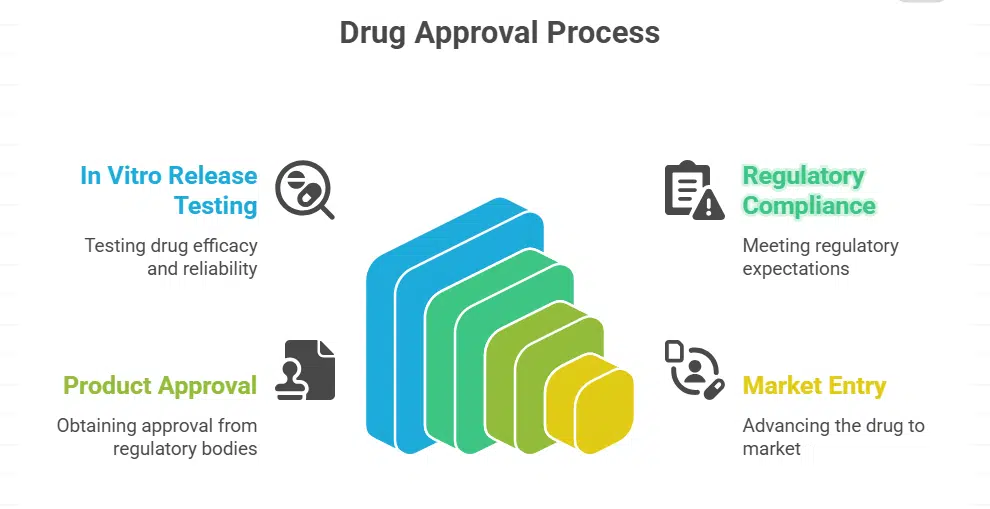
In vitro release testing is an essential aspect of the drug development process. It allows researchers to better understand how a drug would behave once released under normal laboratory conditions. Such testing helps ensure the formulation, safety, and effectiveness of pharmaceuticals but must also be done properly to ensure that pharmaceuticals meet the standards before being given to patients.
Understanding In Vitro Release Testing
In vitro release testing (IVRT), is a test used to measure the release of a drug from its dosage form—a tablet or capsule, for example. This happens inside laboratory-controlled conditions that make it much easier for scientists to establish the speed and overall measure of how much of the medicine dissolves. Understanding these properties allows researchers to get an idea of how that drug will behave after it has been administered.
Without shaking, testing like this is essential for both original and generic drugs. This allows for the active ingredients to be released at a consistent and predictable rate. Such consistency is paramount to retaining efficacy and safety of the therapy.
Improving Drug Formulation
One of in vitro release testing’s main benefits is that it is important for optimizing drug formulations. Researchers can experiment with various formulations to determine which one gives the optimum release profile. Modifying aspects such as the composition of the tablet or excipients can improve the performance of drug substances.
Using this, pharmaceutical companies will formulate the drug so that it reaches the target site in the body properly. This optimization process can result in higher bioavailability, where a greater proportion of the administered drug reaches the systemic circulation, thus improving efficacy.
Ensuring Safety and Efficacy
Drug development focuses on safety and efficacy. In vitro release testing helps with both. By studying how a drug centimeters and diffuses, it can detect potential issues at an early stage. This timely detection enables modifications in advance of clinical trials.
Another place where testing helps is when new formulations are created; they are tested in comparison with already existing formulations. But for generics, it is critical to mimic the release profile that was original to the product. This ensures that the generic will be as effective and as safe as the brand-name drug for all patients.
Regulatory Compliance
One requirement for regulatory bodies to approve new drugs is to conduct in vitro release testing. Such tests provide valuable information about a drug’s efficacy and reliability. However, pharmaceutical companies must meet these regulatory expectations to obtain product approval and advance to market.
Compliance with these requirements not only assures compliance but also builds trust with the HCPs and patients. Stable and dependable release information reinforces its overall legitimacy as a pharmaceutical.
Reducing Development Costs
Developing drugs is expensive and time-consuming. Conducting in vitro release testing is not only a good way to prevent problems early on that may prove to be costly, as an article by the Chalmers University of Technology suggests. While companies invest heavily in drug development, they can avoid expensive setbacks during clinical trials by tackling formulation challenges upfront.
Such an action-oriented approach paves the way for a prompt development process and, thereby, faster changes and enhancements. This allows the business to better serve patients who can benefit from effective therapies, providing swift value to the patients.
Conclusion
In vitro release testing has become an integrated part of modern drug development. It is useful for drug formulation, safety, and efficacy evaluation, as well as regulatory applications, as it provides insights into drug behaviors. It also fosters innovation and lowers the cost of development. Consequently, in vitro release testing makes a major impact on the final delivery of innovative and improved medicinal products to the patient population across the globe via these inputs.