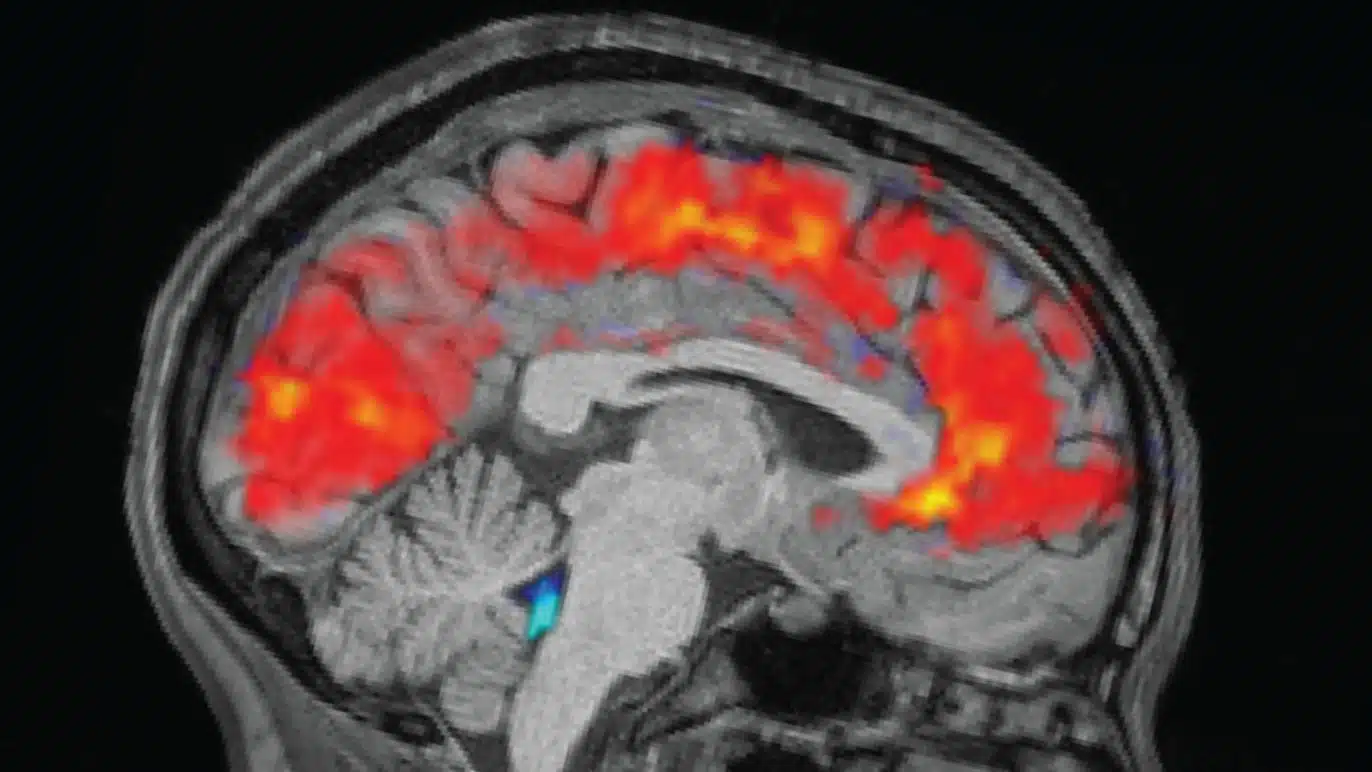
A groundbreaking study reveals that functional magnetic resonance imaging (fMRI), a cornerstone of modern brain research, misinterprets neural activity in about 40% of cases. Researchers from the Technical University of Munich (TUM) and Friedrich-Alexander-University Erlangen-Nuremberg (FAU) discovered that increased fMRI signals often correspond to reduced brain activity, upending a key assumption behind tens of thousands of studies worldwide. This finding, published in Nature Neuroscience, urges a reevaluation of fMRI data in fields from psychiatry to neurology.
The Core Discovery: fMRI’s Hidden Flaw
In the study, scientists tested over 40 healthy participants using tasks like mental arithmetic and autobiographical memory recall, which reliably trigger known brain activations. Traditional fMRI measures the blood-oxygen-level-dependent (BOLD) signal, assuming higher neural activity always boosts blood flow to deliver more oxygen. However, by pairing fMRI with a novel quantitative MRI technique that directly tracks oxygen consumption, the team found stark mismatches: in roughly 40% of cases, elevated BOLD signals appeared in regions with lowered neural activity, while diminished signals showed up where activity spiked.
Dr. Samira Epp, the study’s first author, highlighted the contradiction: “This contradicts the long-standing assumption that increased brain activity is always accompanied by an increased blood flow to meet higher oxygen demand.” Instead, many brain areas satisfy extra energy needs by extracting more oxygen from existing blood supplies, improving efficiency without ramping up perfusion. These “discordant” voxels—about 40% of those with significant BOLD changes—clustered notably in the default mode network, a system active during introspection and mind-wandering.
The implications ripple across neuroscience. PD Dr. Valentin Riedl, now a professor at FAU, noted that this efficiency shift explains why BOLD signals can invert: regions pulling more oxygen from steady blood flow produce misleading readouts. Quantitative analyses confirmed that discordant areas relied on changes in oxygen extraction fraction, while “concordant” ones (the remaining 60%) followed the classic blood flow model. This dual mechanism, varying by task and region, exposes fMRI’s overreliance on vascular proxies for neural truth.
How fMRI Works—and Where It Falls Short
Functional MRI, developed in the early 1990s, detects brain activity indirectly via hemodynamic responses: neurons firing demand more oxygen-rich blood, causing localized blood volume and oxygenation shifts. The BOLD contrast, introduced by Seiji Ogawa in 1990, captures these deoxyhemoglobin dips as brighter signals on scans. Over three decades, fMRI has mapped everything from decision-making circuits to language processing, powering over 40,000 publications annually at its peak.
Yet reliability concerns have simmered for years. Earlier critiques pointed to low test-retest consistency, with intraclass correlations averaging a “fair” 0.5 in adult studies—meaning scans often fail to replicate even days apart. Small sample sizes (typically under 30 participants) amplify biases, inflating reported activation foci beyond what meta-analyses confirm. A 2013 analysis found studies with fewer than 40 subjects claimed suspiciously high activation counts, hinting at selective reporting.
This new research drills deeper, proving BOLD’s neurovascular coupling isn’t universal. In efficient regions, oxygen extraction fraction (OEF) adjusts dynamically: higher neural demand triggers tighter oxygen scavenging from hemoglobin, stabilizing blood flow but flipping BOLD signals. Animal precedents hinted at this—rodent studies showed inverted BOLD in vascular-altered tissues—but human confirmation is seismic. Epp’s team quantified it precisely, revealing discordant voxels differ in baseline OEF, making them prone to inversion during tasks.
Shaking Foundations in Brain Disorder Research
fMRI’s flaws hit hardest in clinical neuroscience, where BOLD changes proxy for pathology. Depression studies often show hypoactivation in reward circuits like the ventral striatum, interpreted as neural deficits—but these may reflect vascular tweaks from aging or medication. Alzheimer’s research flags prefrontal and hippocampal signal drops as atrophy markers, yet Riedl warns: “In patient groups with vascular changes, measured values may primarily reflect vascular differences rather than neuronal deficits.”
Consider aging brains: vascular stiffening reduces flow responsiveness, mimicking underactivation on fMRI despite intact neurons. Psychiatric trials for schizophrenia or anxiety similarly equate BOLD lapses with dysfunction, but 40% discordance suggests many “underactive” zones might actually hum with efficient oxygen use. A 2022 meta-analysis on divergent thinking already questioned fMRI’s granularity, finding inconsistent correlates for creativity. Longitudinal adolescent scans report ICCs below 0.4 for some tasks, classifying them as “poor.”
| Disorder | Common fMRI Finding | Potential Reinterpretation |
|---|---|---|
| Depression | Reduced BOLD in prefrontal cortex | Vascular inefficiency, not neural loss |
| Alzheimer’s | Hippocampal hypoactivation | Oxygen extraction shifts masking activity |
| Aging | Global BOLD decline | Flow decoupling from true metabolism |
| Schizophrenia | Auditory cortex hyperactivity | Artifact from baseline OEF variations |
This table illustrates how reinterpretation could pivot treatments from neural “boosters” to vascular enhancers like exercise or vasodilators. Past over-reliance risks misguided therapies; a 2017 review debunked blanket fMRI invalidity claims but admitted statistical pitfalls persist.
Historical Context: fMRI’s Rocky Road to Dominance
fMRI exploded post-1991, blending MRI’s safety with PET’s metabolic insights minus radiation. Early wins included mapping visual cortex (1992) and motor areas, fueling the Decade of the Brain. By 2000, it decoded lie detection and pain matrices, spawning commercial ventures like neuro-marketing.
Skepticism brewed early. A 2013 bias study revealed underpowered designs (median n=12) overreported foci, with small studies claiming >40 activations versus meta-analyses’ <25. Reliability meta-analyses pegged average ICC at 0.5, “fair” per Cicchetti scales, with adolescents faring worse. The 2016 Eklund paper ignited debate, showing cluster-correction flaws inflated false positives 3,750-fold.
Critics like Craig Bennet (dead salmon scan) satirized spatial biases, scanning a dead fish to “activate” on uncorrected data. Yet fMRI endures for whole-brain, non-invasive power. This TUM-FAU work doesn’t invalidate it wholesale but demands quantitative upgrades—tracking absolute oxygen metabolism over relative BOLD.
Pioneers like Ogawa foresaw limits: BOLD assumes tight coupling, ignoring OEF variability. Recent advances, like high-res 7T scanners, amplify signals but inherit the flaw. The study’s tasks—arithmetic taxing parietal lobes, memory engaging default networks—mirrored real paradigms, ensuring broad relevance.
Expert Reactions and Broader Neuroscience Debate
Online forums buzz with implications. Hacker News threads dissect the 40% figure, questioning if default mode dominance signals introspection’s unique energetics. Valentin Riedl advocates energy-based models: future scans displaying oxygen watts consumed, not flow proxies.
Neuroscience News framed it as a “core assumption overturned,” predicting reinterpretations in thousands of papers. FAU’s Epp calls for hybrid protocols: standard BOLD plus quantitative OEF mapping. Funding from the European Research Council (ERC Starting Grant) underscores rigor, conducted at TUM’s Neuro-Head Center.
Skeptics note limitations: healthy cohorts may not generalize to patients; tasks were basic, not ecologically complex. Still, animal parallels bolster claims—rat studies mirror human discordance in vascular stress. Precision neuroscience pushes back, arguing single-subject reliabilities hover low anyway (ICC<0.4).
| Metric | Traditional fMRI | Quantitative Upgrade |
|---|---|---|
| Measures | Relative BOLD change | Absolute oxygen consumption |
| Reliability | ICC ~0.5 (fair) | Direct metabolism tracking |
| Clinical Pitfall | Vascular confounds | Neuron-specific energy use |
| Future Potential | Activation maps | Energy metabolism atlases |
This shift could refine AI-brain interfaces or drug trials, where BOLD misreads stall progress.
Path Forward: Redefining Brain Imaging Standards
Researchers propose “quantitative functional MRI”: fusing BOLD with OEF for hybrid maps showing true energy budgets. Long-term, energy models could quantify aging’s toll—does a 70-year-old cortex guzzle more watts for the same recall? Patient stratification sharpens: distinguish vascular from neural ills via baseline OEF profiles.
Implementation hurdles loom. Quantitative MRI demands specialized sequences, longer scans (40+ minutes), and post-processing tweaks. Costlier 3T/7T machines limit accessibility, but cloud pipelines could democratize. Reanalyzing archives—tens of thousands BOLD datasets—becomes urgent; open-access repositories like OpenNeuro offer starting points.
Clinical trials pivot: depression interventions targeting “hypoactive” zones might boost flow first, unmasking hidden efficiency. Neurotech firms rethink lie-detectors or neurofeedback, long criticized for BOLD artifacts. Policymakers eye funding: ERC’s bet paid off, signaling more for hybrid tech.
Ethical angles emerge. Overhyped fMRI fueled neuromyths—like 10% brain use—but this curbs overinterpretation. Informed consent evolves: patients learn scans proxy metabolism, not fire directly. Global consortia, like the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA), could standardize quantitative protocols.
Implications for Society and Science Policy
Beyond labs, fMRI shapes courts (lie detection admissibility wanes post-flaws) and education (learning styles debunked). This study fortifies caution: vascular mimics don’t convict or certify. Media literacy surges—headlines like “Brain Scans Lie 40% Time” demand nuance.
Policy-wise, NIH and EU prioritize reliability; post-2020, grants mandate power analyses and test-retest data. TUM-FAU’s open-access paper (Nature Neuroscience, DOI pending) accelerates scrutiny. Young researchers pivot: PhDs blend MRI physics with metabolism modeling.
In sum, this isn’t fMRI’s obituary but evolution. Discordance unveils brain’s thrift—efficient oxygen hacks powering cognition sans floodgates. Neuroscience, long BOLD-bound, marches toward metabolic precision, promising truer portraits of the mind’s energy dance.