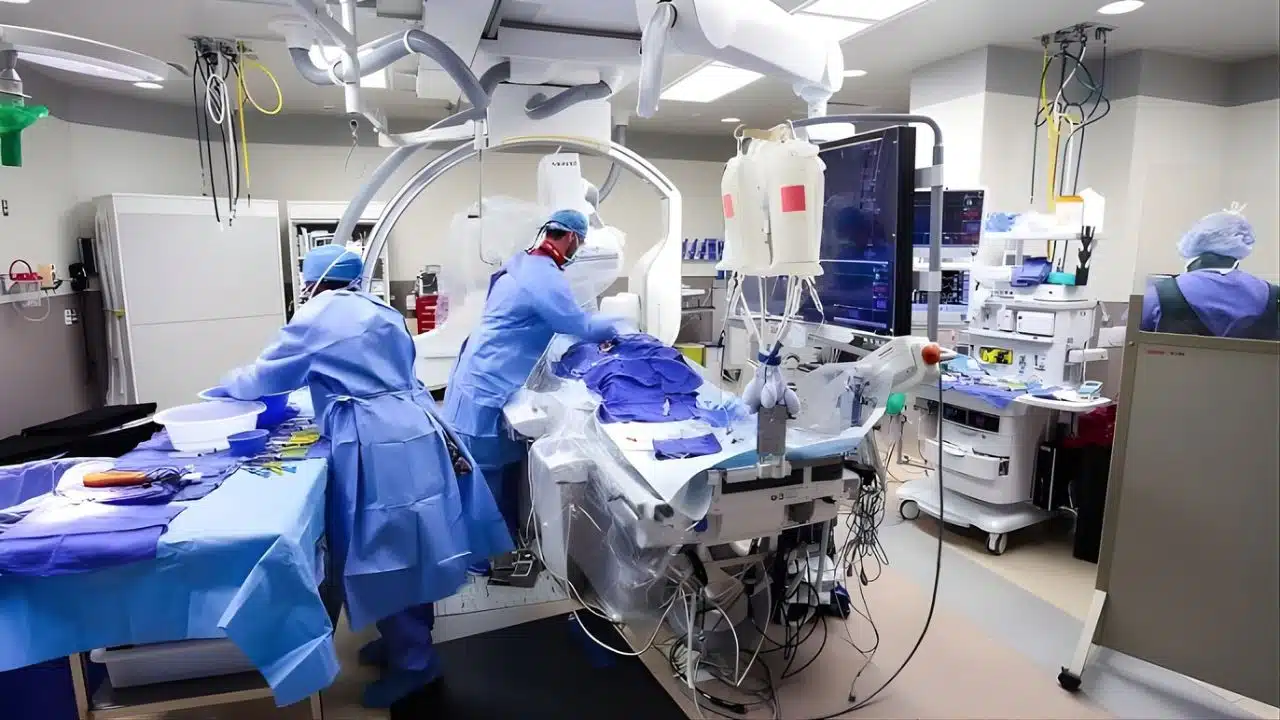
In a watershed moment for Indian healthcare and medical innovation, the All India Institute of Medical Sciences (AIIMS), New Delhi, has successfully completed the country’s first dedicated clinical trial for an indigenous brain stent-retriever. The trial, aimed at treating severe strokes, has demonstrated world-class safety and efficacy, paving the way for affordable, cutting-edge stroke care for millions of Indians.
The groundbreaking study, known as the GRASSROOT Trial (Gravity Stent-Retriever System for Reperfusion of Large Vessel Occlusion Stroke Trial), evaluated the “Supernova” stent-retriever. Developed by Gravity Medical Technology, the device is designed specifically to address the unique anatomical and clinical challenges of stroke patients in India.
The success of this trial marks a significant victory for the “Make in India” initiative in the high-tech medical device sector, a domain long dominated by expensive imported technologies.
A Historic Milestone for Indian Medicine
The GRASSROOT trial is the first instance in India’s medical history where a stroke treatment device has been cleared for routine clinical use based entirely on domestic clinical trial data. Historically, India has relied on devices approved by the US FDA or European CE marks, often without rigorous testing on the local population.
The trial’s results, published this month in the prestigious Journal of NeuroInterventional Surgery (JNIS) — a publication of the British Medical Journal group — revealed that the indigenous device matched, and in some metrics exceeded, global standards.
Dr. Shailesh B. Gaikwad, Professor and Head of the Department of Neuroimaging and Interventional Neuroradiology at AIIMS New Delhi, served as the National Principal Investigator for the trial.
“This trial is a turning point for stroke treatment in India,” Dr. Gaikwad stated. “We have demonstrated that India can not only manufacture world-class medical devices but also generate rigorous clinical evidence to validate them. We no longer need to depend solely on Western data for our patients.”
The GRASSROOT Trial: By the Numbers
The multi-center trial was conducted across eight premier stroke centers in India, with AIIMS New Delhi serving as the national coordinating center. The study enrolled 32 patients suffering from Large Vessel Occlusion (LVO) strokes—a severe form of ischemic stroke where a major artery in the brain is blocked by a blood clot.
The results were striking:
-
High Success Rate: Doctors achieved successful reperfusion (restoration of blood flow) in nearly 94% of patients.
-
Efficiency: In most cases, the blockage was cleared within one or two attempts, eliminating the need for additional “rescue” therapies.
-
Patient Recovery: By the 90-day mark, approximately 50% of the patients had regained functional independence, a critical metric in stroke rehabilitation.
-
Safety Profile: The trial reported low rates of mortality and serious complications, such as brain bleeding, confirming the device’s safety profile is comparable to the best devices currently available globally.
Based on this robust data, the Central Drugs Standard Control Organisation (CDSCO) has approved the Supernova stent-retriever for routine use across India.
The “Supernova”: Tailored for Indian Patients
The device at the heart of this breakthrough, the Supernova stent-retriever, is a mechanical thrombectomy device. Unlike a traditional heart stent that remains in the body to keep an artery open, a stent-retriever is a temporary tool. It is threaded through a catheter from the groin up to the brain, where it enmeshes with the blood clot. The surgeon then pulls the device back, retrieving the clot and restoring blood flow. What makes the Supernova unique is its design philosophy.
“Strokes in India often present differently than in the West,” explained Dr. Ashutosh Jadhav, Chief Scientific Officer at Gravity Medical Technology. “Indian patients are often younger, and their vessel anatomy and clot composition can differ. The Supernova was engineered with inputs from both Indian and international experts to navigate these specific challenges.”
The device features a dual-layer design that optimizes clot integration, allowing for a “first-pass effect”—clearing the vessel in a single attempt. Speed is critical in stroke care, where the mantra is “time is brain.” Every minute a large vessel stroke goes untreated, the average patient loses 1.9 million neurons.
Bridging the Affordability Gap
One of the most profound implications of this development is the potential reduction in treatment costs. Currently, mechanical thrombectomy is the gold standard for treating severe ischemic strokes, but it is prohibitively expensive for the vast majority of Indians. Imported stent-retrievers can cost anywhere between ₹4 lakh to ₹8 lakh, depending on the manufacturer and hospital. This high cost contributes to a massive treatment gap.
“India sees nearly 1.7 million new stroke cases every year,” said Dr. Dileep Yavagal, Professor of Neurology and Neurosurgery at the University of Miami and the Global Principal Investigator of the trial. “However, only about 4,500 of eligible patients actually receive mechanical thrombectomy. The primary barrier is cost.”
With the Supernova device set to be manufactured in India, the cost of the device is expected to drop significantly. While the exact commercial pricing has not yet been released, industry experts anticipate it will be a fraction of the cost of imported alternatives like those from Medtronic or Stryker.
“Affordability is access,” Dr. Yavagal added. “By manufacturing this in India, we are not just lowering a price tag; we are opening the door for tens of thousands of patients who previously would have been left disabled or dead because they couldn’t afford the procedure.”
A “Make in India” Success Story
The development represents a maturing of the Indian medical device ecosystem. Gravity Medical Technology, the company behind the device, describes the CDSCO approval as “more than just a regulatory milestone.”
“This achievement demonstrates that India can design and deliver clinical trials of global significance, accelerating access to advanced therapies while upholding equity,” said Dr. Shashvat M. Desai, Chief Technology Officer at Gravity.
The trial’s success validates the capability of Indian institutions like AIIMS to conduct complex, regulatory-grade clinical research. Dr. Deepti Vibha, Professor of Neurology at AIIMS, emphasized the role of the patients and their families in this achievement.
“Their participation was an act of faith that will now bring faster, more affordable treatments to millions,” Dr. Vibha said. “This is a victory for Indian science and Indian patients alike.”
The Silent Epidemic: Stroke in India
The timing of this innovation is critical. Stroke is rapidly becoming a leading cause of death and disability in India. Unlike in Western nations, where stroke is predominantly a disease of the elderly, in India, nearly 15-20% of strokes occur in people below the age of 40.
The socioeconomic impact of this “young stroke” phenomenon is devastating, as it often strikes the primary breadwinners of families.
The current infrastructure for stroke care in India is heavily skewed towards urban centers. While major metros have comprehensive stroke centers, Tier-2 and Tier-3 cities often lack both the specialists and the affordable technology required for advanced interventions like thrombectomy.
By reducing the cost of the primary consumable—the stent-retriever—hospitals in smaller cities may find it more viable to offer these life-saving procedures, provided they have trained interventional neurologists.
Future Outlook
With regulatory approval secured, Gravity Medical Technology is moving towards mass production of the Supernova system. The company has already used the device to treat over 300 patients in Southeast Asia, but the Indian market remains its primary focus for impact.
Furthermore, the success of the GRASSROOT trial is expected to encourage other Indian med-tech startups to pursue indigenous innovation. It establishes a regulatory pathway that proves domestic trials are a viable, and perhaps superior, route to market for devices intended for the Indian population.
For now, the medical community is celebrating. In the sterile cath labs of AIIMS, where the difference between life and death is measured in millimeters and minutes, doctors finally have a weapon that is world-class, affordable, and proudly made in India.