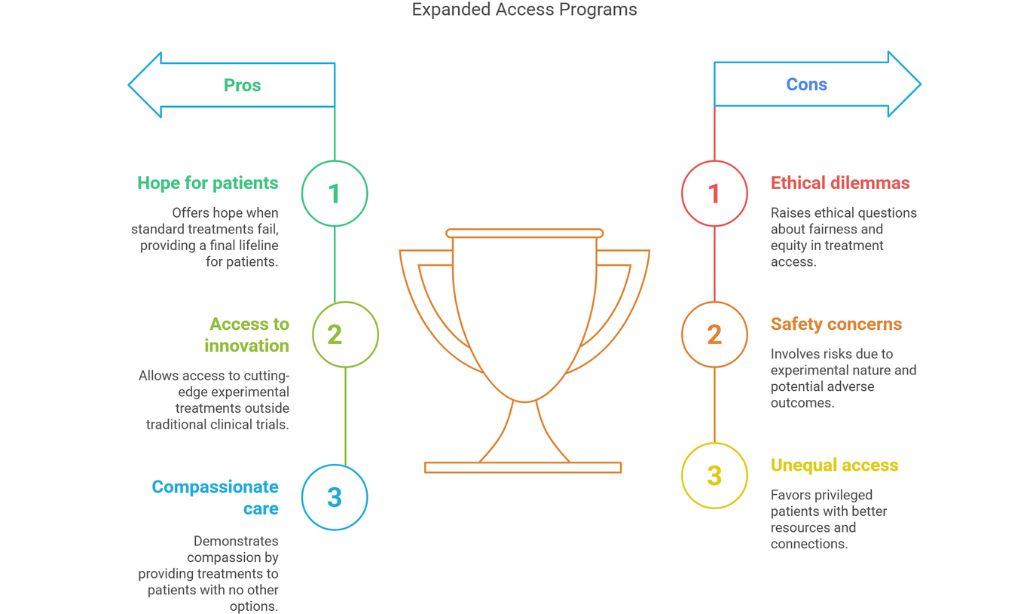
When standard treatments fail and clinical trials aren’t an option, desperately ill patients often find themselves in a medical no-man’s land. For some, expanded access programs offer a final lifeline – a chance to receive experimental treatments not yet approved by regulatory authorities. These programs, also known as compassionate use, represent both profound hope and complicated ethical territory in modern medicine.
Michael Thompson, 42, remembers the day his oncologist told him there were no more conventional options for his aggressive form of brain cancer. “It felt like being handed a death sentence,” he says. “But then they mentioned expanded access, and suddenly there was a door that wasn’t completely closed.”
Expanded access programs allow patients with serious or life-threatening conditions to access investigational drugs, biologics, or medical devices outside the traditional clinical trial framework. For pharmaceutical companies and regulatory bodies like the FDA, these programs walk a delicate line between compassion and caution.
Dr. Sarah Whitman, an oncologist at Memorial Sloan Kettering Cancer Center, explains: “These programs acknowledge a fundamental reality – patients facing terminal illness simply don’t have the luxury of waiting for the standard approval process, which can take years. But we still need safeguards to protect vulnerable patients.”
The path to obtaining an experimental treatment through expanded access is rarely straightforward. Patients must meet specific eligibility criteria, physicians must believe the potential benefits outweigh the risks, and pharmaceutical companies must agree to provide the treatment. The FDA must then review and approve the request, though the agency approves over 99% of the applications it receives.
For Diana Chen, whose 8-year-old daughter Lily has a rare genetic disorder, navigating this process became a full-time job. “We had to become experts overnight,” Chen explains. “I was researching clinical trials, contacting researchers directly, and building relationships with anyone who might help us access this experimental therapy.”
Their persistence paid off when Lily became one of the first children to receive an investigational gene therapy through expanded access. Six months later, her symptoms have stabilized – an outcome the family considers nothing short of miraculous.
Not all expanded access stories end with such positive results. Pharmaceutical companies face difficult decisions about providing unapproved treatments. Limited supplies, manufacturing constraints, and concerns about how adverse outcomes might affect the drug’s eventual approval process all factor into their calculations.
“These are experimental therapies for a reason,” cautions Dr. Robert Califf, former FDA Commissioner. “We’ve seen promising treatments in the lab fail dramatically in humans, sometimes causing harm rather than helping. That’s why the regulatory process exists.”
The ethical dimensions of expanded access extend beyond individual patient outcomes. Questions of fairness and equity loom large. Those with financial resources, education, and connections often navigate the system more successfully than disadvantaged populations.
Arthur Caplan, a bioethicist at NYU Langone Medical Center, has spent decades studying these issues. “We need to acknowledge that expanded access as currently structured favors the privileged,” he says. “The patient who has a doctor at a major academic medical center, who understands the regulatory framework, and who can leverage social media to pressure a company – that patient has advantages others don’t.”
Some advocacy organizations are working to level this playing field. The Reagan-Udall Foundation’s Expanded Access Navigator helps patients and physicians understand the process regardless of their resources or connections. Similarly, patient advocacy groups for specific diseases often develop expertise in expanded access and share it within their communities.
Companies like Janssen Pharmaceuticals have created more standardized expanded access programs to ensure equitable treatment. Their CompAC program utilizes an independent committee of bioethicists, physicians, and patient representatives to review requests, removing some decision-making burden from individual doctors while providing more consistent evaluations.
Recent legislative changes have aimed to improve the system. The Right to Try Act of 2018 created an alternative pathway for patients to access experimental treatments, bypassing FDA review in certain circumstances. However, this approach removes important safety oversight, and many physicians and pharmaceutical companies still prefer the established expanded access process.
For terminally ill patients like Jennifer Martinez, these policy debates take on profound personal significance. Diagnosed with ALS three years ago, Martinez has exhausted standard treatments. “I understand there are no guarantees with experimental therapies,” she says. “But having the chance to try feels like maintaining dignity and agency in the face of this disease.”
The medical community continues to grapple with how best to balance innovation, safety, and compassionate care. Dr. Whitman emphasizes that expanded access should complement, not replace, rigorous clinical trials. “The fastest way to help the most patients is still through well-designed studies that can lead to approved treatments,” she notes.
As medicine advances with breakthrough technologies like gene editing, cell therapies, and precision medicine, expanded access programs will likely become even more significant. These programs represent medicine’s struggle to reconcile its methodical, evidence-based approach with the urgent needs of patients facing terminal illness.
For those patients and their families, expanded access offers something beyond the treatment itself – it offers hope when all conventional options have been exhausted. And for a patient facing their mortality, hope itself can be powerful medicine.





































